Abstract
Background
A growing body of research supports the important role of the TH2 axis in alopecia areata (AA). Dupilumab is a humanized monoclonal antibody against IL-4Rα that downregulates TH2 response. Although efficacy has been shown in clinical trials, real-world data on the use of dupilumab in AA patients is limited.
Objectives
To report on a case series of 10 patients with AA who were treated with dupilumab and provide real-world evidence regarding its efficacy in treating severe AA.
Methods
In this retrospective single-center study, all AA patients treated with dupilumab treatment were included between May 2022 and October 2023. Clinical outcome measures (Severity of Alopecia Tool, SALT) and adverse events (AEs) were analyzed. In addition, a literature review was conducted to summarize the efficacy of AA with dupilumab and the characteristics of patients previously reported in the literature.
Results
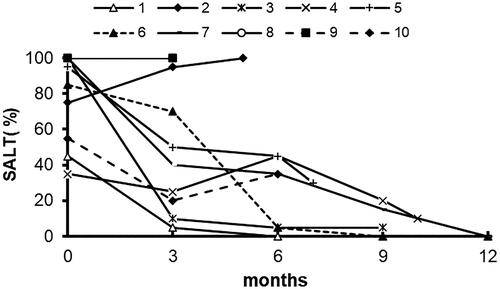
We identified 10 patients with AA who were or are being treated with dupilumab, with a median (range) treatment duration of 8 (3-15) months. Of these, four patients have high serum immunoglobulin E (IgE) levels (≥200IU/ml). The mean (IQR) pretreatment SALT score was 79% (52-100). Seven of 10 patients achieved at least 50% re-growth. Of those who improved, the mean (IQR) percentage change in SALT score at 3 months and the end of follow-up was 57% (29%-89%) and 95% (68-100), respectively. Notably, seven patients (70%) had white hair regrowth, with the white hair slowly decreasing over time and the proportion of pigmented black hair increasing. Dupilumab was well tolerated by all patients. No adverse events were reported.
Conclusions
Overall, our research supports dupilumab as another candidate that possesses potential benefits for AA. High levels of IgE may be not prerequisites for dupilumab’s successful treatment response.
Introduction
Alopecia areata (AA) is a chronic, relapsing, immune-mediated disorder that is characterized by uncontrolled non-scarring hair loss (Citation1). Considering the sociocultural associations between hair and sexuality, health, and youth, AA can have a significant negative impact on quality of life, mental health, and productivity (Citation2,Citation3).
The management of AA in clinical practice is notoriously challenging, with high rates of therapeutic failures or relapses, especially those with severe long-term clinical courses (Citation4,Citation5). While there is growing evidence supporting the effectiveness of Janus kinase (JAK) inhibitors in treating AA, the high rate of recurrence following drug discontinuation has led to prolonged treatment courses and raised concerns about long-term safety (Citation6,Citation7). Moreover, even with a longer treatment period, more than half of patients treated with JAK inhibitors are reported to experience relapse after discontinuing therapy (Citation8). Existing research suggests that JAK inhibitors may not alter the natural course of AA, and our experience is that many patients need treatment beyond 12 months to achieve a longer remission duration (Citation9). There is limited guidance for clinicians on how to manage these patients in the longer term. In addition, despite the great advances and encouraging achievements in AA treatment, JAK inhibitors are still ineffective in nearly 30% of patients (Citation8). Given the risk of deteriorating quality of life, anxiety, and depression with poorly controlled AA, the importance of rapidly and safely mitigating disease activity cannot be overemphasized. As such, there is an urgent need for a disease-modifying therapy to improve the efficacy and prognosis of this disease.
To date, the underlying disease mechanisms in AA are not yet fully elucidated. The traditional view holds that a major role of interferon-γ-mediated Th1-responses, while others highlight Th2-mediated effects and regulatory T-cell (Treg) deficiency (Citation10–12). It has recently been shown that atopic status in AA is a poor prognostic factor and promotes a protracted course of disease (Citation12). In particular, allergen desensitization can reduce the severity of relapsed alopecia areata in dust-mite allergic patients (Citation13). A Phase 2a randomized clinical trial further indicates the possible pathogenic role of the Th2 axis and Th2 targeting in AA patients (Citation14). However, there are conflicting results on the efficacy of dupilumab in treating AA (Citation15). This aroused our interest in exploring whether anti-atopy therapy can treat and potentially improve patient outcomes.
Dupilumab, a humanized monoclonal antibody against IL-4Rα that downregulates TH2 response, is increasingly being used in treatment-refractory atopic dermatitis (AD) (Citation16), which allows us to gain a better understanding of atopy and AA. Considering that patients with longer episodes of severe disease have a poorer prognosis, treating patients early in life and before complete hair loss occurs might substantially increase response rates and reduce relapses. In addition, dupilumab use has accumulated a large bulk of multiyear longitudinal safety data across indications and does not require laboratory monitoring during the working period (Citation17).
Therefore, we hypothesized that patients with AA and atopic status may be the best candidates for dupilumab treatment. Here we present a retrospective study on patients with AA who were treated with dupilumab. Response to treatment and the safety profiles are evaluated. We also conducted an extensive literature review to further determine the main characteristics of patients treated with dupilumab for alopecia or alopecia due to dupilumab treatment.
Methods
Study design and patients
This retrospective study enrolled patients with AA who were treated with dupilumab between May 2022 and October 2023 at the Department of Dermatology in The Xiangya Hospital of Central South University, Changsha, China. The diagnosis of AA was made by dermatologists (W.S. and J.L.) based on clinical characteristics. The inclusion criteria were as follows: (a) patients with a clinical diagnosis of AA; (b) patients suffering from AA who failed to respond to previous topical therapy or were reluctant to use JAK inhibitor or other immunosuppressors (e.g. systemic corticosteroid therapy, cyclosporin, methotrexate) considering side effects. Those lost to follow-up and those with less than 3 months of treatment were also excluded from the analysis.
All patients or their legal representatives provided written informed consent and were aware of off-label dupilumab use. This study was approved by the hospital’s Research Ethics Committee and is by the Declaration of Helsinki.
Therapeutic regimen
Considering the important context of medical insurance reimbursement in China, dupilumab was administered at the scheduled dosage for AD. For adult patients, dupilumab is administered at a loading dose of 600 mg subcutaneously, followed by 300 mg every other week (Q2W) (Citation18). For minors, dose adjustment is recommended depending on body weight (bodyweight ≥5 kg to <15 kg: 200 mg, every 4 weeks; bodyweight ≥15 kg to <30 kg: 300 mg, every 4 weeks, with or without a first loading double dose; bodyweight ≥30 kg to <60 kg: 200 mg, every 2 weeks, with a first loading double dose), and patients could also receive a personalized dosing regimen (Citation16,Citation19–21). Some patients choose to combine topical glucocorticoid therapy because they are used before dupidumab therapy.
Data collection and outcomes
For each patient, demographic and clinical characteristics (age, sex, weight, age of onset, duration of current episode of disease, family history and severity, atopic and non-atopic comorbidities, and previous treatment history) were collected. Effectiveness outcomes (Severity of Alopecia Tool, SALT (Citation22)) were collected at week 0 (baseline) and each follow-up visit. Eczema Area and Severity Index (EASI) score, Worst Pruritus Numerical Rating Scale (NRS), Eyebrow Assessment (Citation23) (EBA, 0–3) score, and Eyelash Assessment (Citation23) (ELA, 0-3) score, Patient Satisfaction with Hair Growth (P-Sat) during treatment were also collected. Safety was evaluated by the incidence of adverse events (AEs) at each visit and significant changes in laboratory values were checked periodically.
Literature review
A comprehensive literature search of PubMed was performed in October 2023. Search terms included: ‘alopecia areata’, ‘alopecia circumscripta’, ‘alopecia totalis’, ‘alopecia universalis’, ‘alopecia celsi’, ‘ophiasis’, ‘nonscarring hair loss’, ‘dupilumab’, ‘SAR231893’, ‘REGN668’, and ‘Dupixent’. In addition, articles including the wording ‘case report’ or ‘case series’ were added. All the sourced articles were full-text reviewed to ensure that the contents were relevant to the study. Only English articles categorized as case reports and case series were included.
Results
Of 11 patients with AA who were or are being treated with dupilumab, 10 patients met the inclusion criteria. The median (range) treatment duration of the 10 patients was 8 (3–15) months. There were 2 men (20%) and 8 women (80%), with a median age at time of treatment of 10 (1–21) years. The mean (SD) pretreatment SALT score was 79% (IQR 52-100), with 2 patients having alopecia totalis, and 5 patients having alopecia universalis (). 7 patients had AD, and 2 patients had allergic rhinitis. Of these, four patients have high serum immunoglobulin E (IgE) levels (≥200IU/ml) and three patients have elevated eosinophil cell counts at baseline; The enrolled patients had undergone various therapies for AA, including corticosteroids [topical (n = 4), and oral (n = 5)], topical minoxidil (n = 5) or tofacitinib(n = 1), but had suboptimal treatment response.
Table 1. Characteristics of alopecia areata patients treated with dupilumab.
Seven of 10 patients achieved at least 50% re-growth (, ). Of those who improved, the mean (IQR) percentage change in SALT score at 3 months and the end of follow-up was 57% (29%-89%) and 95% (68-100), respectively. Three patients (patients 1, 6, 7) showed complete hair regrowth (SALT score =0) with an average treatment duration of 8 months, one of them on dupilumab monotherapy (). However, three patients (patients 2, 8, 9) had no regrowth despite being on dupilumab for 3 to 5 months, and patient 2 worsened on dupilumab and was concomitantly on topical corticosteroids.
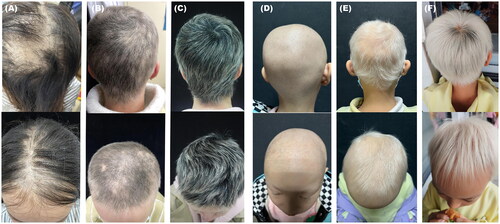
Figure 2. Scalp pictures of two patients. A-C show scalp hair at baseline, month 6, and month 9 visits in patient 1 on dupilumab. D-F show scalp hair at baseline, month 3, and month 9 visits in patient 7 on dupilumab.
Partial or complete loss of eyebrows and eyelashes was observed in 7 (70%) and 4 (40%) patients, respectively. None of these patients achieved ≥ 1 grade improvement in eyelash and eyebrow assessment at month 3, and only three patients and one patient achieved ≥ 1 grade improvement in eyelash and eyebrow assessment at month 6 compared to baseline.
We also observed that seven patients had white vellus hair regrowth and terminal hair appearing on the scalp eyelashes or eyebrows. During treatment, the proportion of white hair continuously decreased and the proportion of pigmented black hair increased.
Overall, the majority of patients (6, 60%) expressed satisfaction with the effectiveness of dupilumab. Four reported being ‘very satisfied’ and two were ‘moderately satisfied.’ All patients, including those with no regrowth, showed significant clinical improvement in their AD and a significant reduction in their EASI and NRS scores.
Dupilumab was well tolerated by all patients. No adverse events were reported. Six patients continued treatment. The remaining four patients stopped therapy after the duration of treatment of 3-6 months, because of loss of efficacy or delayed onset of effect. After withdrawal of dupilumab, patient 2 chose to continue topical corticosteroids in combination with 0.03% tacrolimus. Surprisingly, Significant hair regrowth to SALT score 70 after 4 months of treatment; Patient 3 transitioned to Baricitinib for five months but did not show any improvement.
Literature review
The literature search in PubMed yielded 86 articles. After the screening process, a total of 45 articles were labeled as eligible. In all, 22 patients (15 men and 7 women, 15–63 year age range) who developed or worsened alopecia following treatment with dupilumab have been reported in the literature to date; A total of 40 patients (21 men and 19 women, 4–65 year age range) with AA reported a benefit with dupilumab (defined as greater than 30% change in SALT score), while 21patients (7 men and 14 women, 4–62 year age range) with AA who received dupilumab did not respond. and Citation3 summarize the main clinical characteristics of those patients.
Table 2. Summary of the literature on dupilumab treatment of alopecia areata.
Table 3. New-onset or worsening alopecia with dupilumab.
Dupilumab-induced alopecia is a really rare adverse effect, and most patients (15/22) deny a previous history of AA. The time lapse between starting dupilumab and the onset of AA ranged between 1 week and 112 weeks. Interestingly, patients with alopecia induced by dupilumab showed a favorable prognosis with some experiencing complete spontaneous remission even without discontinuation of the drug. Therefore, we cannot help suspecting that the ‘induction’ of AA in dupilumab-treated AD patients may be random. Whether the induction of AA resulted from dupilumab-induced activation of alternative immune pathways or occurred naturally remains confused.
Although numerous case reports suggest the beneficial effects of dupilumab on AA, the real efficacy of this approach is still debated. Additionally, publication bias for ‘positive’ studies should be taken into account. Similar to our patients, the majority had previously been unresponsive to various treatments for AA, including topical and systemic corticosteroids, cyclosporine, methotrexate, minoxidil, or contact immunotherapy. Of these patients, 40 responded to dupilumab and 53% of patients achieved a 100% improvement in SALT score, with a median (range) treatment duration of 7.5 (3-36) months. There were 21 cases of AA not responding to dupilumab after 2-16 months of treatment, but all patients had clinical improvement in their AD and asthma. In most patients, higher doses of dupilumab and longer treatment duration were associated with a better response in AA. Contrary to data reported in phase IIa trial (Citation14), the results did not show that IgE levels can predict dupilumab response. In patients with IgE ≥ 200 IU/ml and normal IgE, 50% (n = 10) and 100% (n = 2) achieved SALT80, respectively. Similarly, no sex-based differences were observed in the AA response to dupilumab. Among these reports, the most common side effect was mild injection site reactions, and no one discontinued treatment due to side effects.
Discussion
Although dupilumab has shown to be effective in the treatment of several dermatological conditions and gained popularity for AA treatment in recent years, most reports regarding this subject are case reports and are rarely sufficient. This study reports real-world data on a cohort of 10 Chinese patients with moderate to severe AA treated with dupilumab. The outcomes of this study offered an optional, effective, and safe alternative treatment for AA.
A recent phase IIa clinical trial, including 40 subjects receiving weekly administered dupilumab 300 mg, and 20 subjects receiving a placebo, provided preliminary evidence of dupilumab efficacy in AA (Citation14). In the treatment group, responders who achieved SALT 30, SALT 50, SALT 75, and SALT 90 responses accounted for 17.5%, 10%, 5%, and 2%, respectively, at week 24. The response rates continue to improve among patients who reached week 48, with 32.5%, 22.5%, 15%, and 10% of SALT 30/50/75/90, respectively. Although the results of our study and the recent Phase IIa trial cannot be directly compared, our data also indicate a favorable trend of dupilumab in hair regrowth, even in patients with normal IgE levels. Notably, our patients were treated with dupilumab at the scheduled dosage for AD, which was much lower than the dose used in clinical trials and may have contributed to the ineffective or suboptimal response in some patients. The overall response rate was acceptable, though not ideal compared with JAK inhibitors, suggesting that dupilumab may be an effective supplement rather than the first choice for systemic therapy for AA.
While the precise mechanism of action of dupilumab in AA remains unknown, there are several plausible explanations for the different response profiles observed. Firstly, it has been hypothesized that some individuals with AA and atopic status may exhibit more Th2 skewing, which inhibits Treg function and consequently promotes TH1 response, resulting in follicular immune privilege disruption. Prior reports have suggested that patient selection based on baseline serum IgE levels may improve treatment results; however, that is not a fact that we observed, which indicates high levels of IgE are not prerequisites for dupilumab’s successful treatment response. On the contrary, patients without atopic comorbidities may have a shift of Th1/Th2 balance to Th1. Thus, the down-regulation of Th2 after dupilumab use may result in an abrupt skewing of Th1, promoting the pathogenesis of AA. However, some researchers argue that the reported improvement of AA in dupilumab-treated AD patients is random and AA is a highly heterogeneous disease, with different patient populations displaying diverse immune events resulting in a shared clinical phenotype. We are inclined to the TH2 skewing theory and tried to find clinical features or relevant biomarkers in the treatment response population. In addition, we also found that the regenerated hairs after dupilumab were mostly white and had delayed pigment regeneration. A detailed discussion of this phenomenon has been recently published by Yan X, et al. (Citation52)
Finally, the overall favorable adverse effect profile of dupilumab bears highlighting. Nasopharyngitis, upper respiratory tract infection, conjunctivitis, headache, oral herpes, and injection-site reactions were the most common adverse effects noted in a recent 4-year open-label study of dupilumab in patients with AD—notably, new or reactivated tuberculosis (TB) infection were not observed (Citation24). Thus, dupilumab may be an attractive option for those patients with refractory AA with risk factors for TB that would typically preclude the use of JAK inhibitors or other immunosuppressants, after which efficacious treatment options are generally lacking. Moreover, an additional advantage of dupilumab lies in its economic cost and convenience, which is as low as 50% of the expenditures of baricitinib per month after reimbursement and supports its self-administration at home.
The small sample size, retrospective nature, and no comparator are limitations of our study. However, our research shows that patients with AA benefit from dupilumab, which greatly complements the current treatment paradigm of alopecia areata.
In conclusion, we present here a cohort of 10 Chinese patients with moderate to severe AA treated with dupilumab and summarize the characteristics of patients previously reported in the literature, suggesting it may be considered as an alternative therapy for AA. Further larger studies are needed to assess the effectiveness of dupilumab for AA and the relevant prognostic factors.
Author contributions
All authors participated in clinical data collection. JD-H, J-J, and TT-L drafted the manuscript. Corresponding authors W-S and J-L read and revised the manuscript. All authors contributed to the article and approved the submitted version.
IRB approval status
Reviewed and approved by the institutional research ethics boards of Xiangya Hospital, Central South University (Changsha, China); approval number: 202312240. This study was also conducted in adherence to the STROBE guidelines.
Funding sources
None.
Disclosure statement
None declared.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, [WS].
References
- Dainichi T, Iwata M, Kaku Y. Alopecia areata: what’s new in the epidemiology, comorbidities, and pathogenesis? J Dermatol Sci. 2023;112(3):1–11. doi: 10.1016/j.jdermsci.2023.09.008. From NLM.
- Muntyanu A, Gabrielli S, Donovan J, et al. The burden of alopecia areata: a scoping review focusing on quality of life, mental health and work productivity. J Eur Acad Dermatol Venereol. 2023;37(8):1490–1520. From NLM. doi: 10.1111/jdv.18926.
- Lauron S, Plasse C, Vaysset M, et al. Prevalence and odds of depressive and anxiety disorders and symptoms in children and adults with alopecia areata: a systematic review and meta-analysis. JAMA Dermatol. 2023;159(3):281–288. From NLM. doi: 10.1001/jamadermatol.2022.6085.
- Lintzeri DA, Constantinou A, Hillmann K, et al. Alopecia areata - Current understanding and management. J Dtsch Dermatol Ges. 2022;20(1):59–90. From NLM. doi: 10.1111/ddg.14689.
- Tosti A. Alopecia areata: the clinician and patient voice. J Drugs Dermatol. 2023;22(10):967–975. From NLM. doi: 10.36849/jdd.Sf396143.
- Liu M, Gao Y, Yuan Y, et al. Janus kinase inhibitors for alopecia areata: a systematic review and Meta-Analysis. JAMA Netw Open. 2023;6(6):e2320351. From NLM. doi: 10.1001/jamanetworkopen.2023.20351.
- Egeberg A, Linsell L, Johansson E, et al. Treatments for moderate-to-severe alopecia areata: a systematic narrative review. Dermatol Ther (Heidelb). 2023;13(12):2951–2991. From NLM. doi: 10.1007/s13555-023-01044-5.
- Yan D, Fan H, Chen M, et al. The efficacy and safety of JAK inhibitors for alopecia areata: a systematic review and meta-analysis of prospective studies. Front Pharmacol. 2022;13:950450. From NLM. doi: 10.3389/fphar.2022.950450.
- Huang J, Deng S, Li J, et al. Drug survival and long-term outcome of tofacitinib in patients with alopecia areata: a retrospective study. Acta Derm Venereol. 2023;103:adv13475. From NLM. doi: 10.2340/actadv.v103.13475.
- Sterkens A, Lambert J, Bervoets A. Alopecia areata: a review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med. 2021;21(2):215–230. From NLM. doi: 10.1007/s10238-020-00673-w.
- Renert-Yuval Y, Pavel AB, Del Duca E, et al. Scalp biomarkers during dupilumab treatment support Th2 pathway pathogenicity in alopecia areata. Allergy. 2023;78(4):1047–1059. From NLM. doi: 10.1111/all.15561.
- Chen W, Li S, Cai X, et al. Association between alopecia areata and atopic dermatitis: current evidence. J Eur Acad Dermatol Venereol. 2023;37(8):1447–1673, e945–e1088. doi: 10.1111/jdv.19044.
- Zeng Z, Li S, Ye Y, et al. Allergen desensitization reduces the severity of relapsed alopecia areata in dust-mite allergic patients. Exp Dermatol. 2023;32(7):1108–1119. From NLM. doi: 10.1111/exd.14819.
- Guttman-Yassky E, Renert-Yuval Y, Bares J, et al. Phase 2a randomized clinical trial of dupilumab (anti-IL-4Rα) for alopecia areata patients. Allergy. 2022;77(3):897–906. From NLM. doi: 10.1111/all.15071.
- Marks DH, Mesinkovska N, Senna MM. Cause or cure? Review of dupilumab and alopecia areata. J Am Acad Dermatol. 2023;88(3):651–653. From NLM. doi: 10.1016/j.jaad.2019.06.010.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;10356;400(10356):908–919. doi: 10.1016/s0140-6736(22)01539-2.From NLM.
- Xu Y, Guo L, Li Z, et al. Efficacy and safety profile of dupilumab for the treatment of atopic dermatitis in children and adolescents: a systematic review and meta-analysis. Pediatr Dermatol. 2023;40(5):841–850. From NLM. doi: 10.1111/pde.15398.
- Chun PIF, Lehman H. Current and future monoclonal antibodies in the treatment of atopic dermatitis. Clin Rev Allergy Immunol. 2020;59(2):208–219. From NLM. doi: 10.1007/s12016-020-08802-9.
- Spekhorst LS, Bakker D, Drylewicz J, et al. Patient-centered dupilumab dosing regimen leads to successful dose reduction in persistently controlled atopic dermatitis. Allergy. 2022;77(11):3398–3407. From NLM. doi: 10.1111/all.15439.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. From NLM. doi: 10.1001/jamadermatol.2019.3336.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282–1293. From NLM. doi: 10.1016/j.jaad.2020.06.054.
- Olsen EA, Roberts J, Sperling L, et al. Objective outcome measures: collecting meaningful data on alopecia areata. J Am Acad Dermatol. 2018;79(3):470–478.e3. e473 From NLM. doi: 10.1016/j.jaad.2017.10.048.
- Wyrwich KW, Kitchen H, Knight S, et al. Development of clinician-reported outcome (ClinRO) and patient-reported outcome (PRO) measures for eyebrow, eyelash and nail assessment in alopecia areata. Am J Clin Dermatol. 2020;21(5):725–732. From NLM. doi: 10.1007/s40257-020-00545-9.
- Beck LA, Deleuran M, Bissonnette R, et al. Dupilumab provides acceptable safety and sustained efficacy for up to 4 years in an Open-Label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2022;23(3):393–408. From NLM. doi: 10.1007/s40257-022-00685-0.
- Darrigade AS, Legrand A, Andreu N, et al. Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br J Dermatol. 2018;179(2):534–536. From NLM. doi: 10.1111/bjd.16711.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154(11):1358–1360. From NLM. doi: 10.1001/jamadermatol.2018.2976.
- Uchida H, Kamata M, Watanabe A, et al. Dupilumab improved alopecia areata in a patient with atopic dermatitis: a case report. Acta Derm Venereol. 2019;99(7):675–676. From NLM. doi: 10.2340/00015555-3183.
- Smogorzewski J, Sierro T, Compoginis G, et al. Remission of alopecia universalis in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2019;5(2):116–117. From NLM. doi: 10.1016/j.jdcr.2018.11.007.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5(2):111–112. From NLM. doi: 10.1016/j.jdcr.2018.11.006.
- Patruno C, Napolitano M, Ferrillo M, et al. Dupilumab and alopecia: a janus effect. Dermatol Ther. 2019;32(5):e13023. From NLM. doi: 10.1111/dth.13023.
- Ludriksone L, Elsner P, Schliemann S. Simultaneous effectiveness of dupilumab in atopic dermatitis and alopecia areata in two patients. J Dtsch Dermatol Ges. 2019;17(12):1278–1280. From NLM. doi: 10.1111/ddg.13990.
- Call JE, Sahni S, Zug KA. Effectiveness of dupilumab in the treatment of both atopic dermatitis and alopecia universalis. Clin Case Rep. 2020;8(8):1337–1339. From NLM. doi: 10.1002/ccr3.2915.
- Ushida M, Ohshita A, Arakawa Y, et al. Dupilumab therapy rapidly improved alopecia areata associated with trichotillomania in an atopic dermatitis patient. Allergol Int. 2020;69(3):480–482. From NLM. doi: 10.1016/j.alit.2020.02.009.
- Magdaleno-Tapial J, Valenzuela-Oñate C, García-Legaz-Martínez M, et al. Improvement of alopecia areata with dupilumab in a patient with severe atopic dermatitis and review the literature. Australas J Dermatol. 2020;61(2):e223–e225. From NLM. doi: 10.1111/ajd.13208.
- Harada K, Irisawa R, Ito T, et al. The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: a case series of seven patients. Br J Dermatol. 2020;183(2):396–397. From NLM. doi: 10.1111/bjd.18976.
- Zhu GA, Kang KJW, Chen JK, et al. Inflammatory alopecia in patients on dupilumab: a retrospective cohort study at an academic institution. J Eur Acad Dermatol Venereol. 2020;34(4):e159–e161. From NLM. doi: 10.1111/jdv.16094.
- Babino G, Fulgione E, D’Ambra I, et al. Rapid hair regrowth induced by dupilumab in a patient affected by alopecia totalis of 28 years’ duration: clinical and dermoscopic features. Dermatol Ther. 2020;33(4):e13582. From NLM. doi: 10.1111/dth.13582.
- Gruenstein D, Malik K, Levitt J. Full scalp hair regrowth in a 4-year-old girl with alopecia areata and atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6(12):1286–1287. From NLM. doi: 10.1016/j.jdcr.2020.10.010.
- Szekely S, Vaccari D, Salmaso R, et al. Onset of schamberg disease and resolution of alopecia areata during treatment of atopic dermatitis with dupilumab. J Investig Allergol Clin Immunol. 2021;31(1):65–66. From NLM. doi: 10.18176/jiaci.0541.
- Muto J, Yoshida S, Doi C, et al. Dupilumab treatment of atopic dermatitis leading to successful treatment of alopecia universalis: a Japanese case report. J Dermatol. 2021;48(2):e72–e73. From NLM. doi: 10.1111/1346-8138.15631.
- McKenzie PL, Castelo-Soccio L. Dupilumab therapy for alopecia areata in pediatric patients with concomitant atopic dermatitis. J Am Acad Dermatol. 2021;84(6):1691–1694. From NLM. doi: 10.1016/j.jaad.2021.01.046.
- Abercrombie M, Aleshaki J, Fivenson D. Ophiasis treated with dupilumab. JAAD Case Rep. 2021;16:1–4. From NLM. doi: 10.1016/j.jdcr.2021.07.029.
- Choe S, Newman EM. Time to loss of response for dupilumab in ophiasis-pattern alopecia areata. JAAD Case Rep. 2021;15:133–136. From NLM. doi: 10.1016/j.jdcr.2021.07.025.
- Alotaibi L, Alfawzan A, Alharthi R, et al. Improvement of atopic dermatitis and alopecia universalis with dupilumab. Dermatol Reports. 2022;14(2):9359. From NLM. doi: 10.4081/dr.2022.9359.
- Cho SK, Craiglow BG. Dupilumab for the treatment of alopecia areata in children with atopic dermatitis. JAAD Case Rep. 2021;16:82–85. From NLM. doi: 10.1016/j.jdcr.2021.07.015.
- Romagnuolo M, Barbareschi M, Tavecchio S, et al. Remission of alopecia universalis after 1 year of treatment with dupilumab in a patient with severe atopic dermatitis. Skin Appendage Disord. 2022;8(1):38–41. From NLM. doi: 10.1159/000517832.
- Napolitano M, Cantelli M, Potestio L, et al. Clinical, trichoscopic and in vivo reflectance confocal microscopy evaluation of alopecia areata in atopic dermatitis patients treated with dupilumab. J Eur Acad Dermatol Venereol. 2022;36(7):e561–e563. From NLM. doi: 10.1111/jdv.18029.
- McFeely O, Blasco MC, Doyle C, et al. I feel like a new woman’: atopic dermatitis and alopecia areata treated successfully by dupilumab. Clin Exp Dermatol. 2023;48(3):266–267. From NLM. doi: 10.1093/ced/llac088.
- Visconti MJ, Richardson A, LaFond AA. Dupilumab as a therapeutic approach in alopecia universalis. Cutis. 2022;110(5):E7–e8. From NLM. doi: 10.12788/cutis.0659.
- Seo T, Izumi K, Kawamura T, et al. A case of atopic dermatitis-associated dupilumab-resistant alopecia totalis successfully treated with baricitinib. Dermatol Ther. 2022;35(12):e15953. From NLM. doi: 10.1111/dth.15953.
- Kulkarni M, Rohan CA, Travers JB, et al. Long-Term efficacy of dupilumab in alopecia areata. Am J Case Rep. 2022;23:e936488. From NLM. doi: 10.12659/ajcr.936488.
- Yan X, Tayier M, Cheang ST, et al. Hair repigmentation and regrowth in a dupilumab-treated paediatric patient with alopecia areata and atopic dermatitis: a case report. Ther Adv Chronic Dis. 2023;14:20406223231191049. From NLM. doi: 10.1177/20406223231191049.
- Cai L, Wei Y, Zhao M, et al. Case report: dupilumab therapy for alopecia areata in a 4-year-old patient resistant to baricitinib. Front Med (Lausanne). 2023;10:1253795. From NLM. doi: 10.3389/fmed.2023.1253795.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4(2):143–144. From NLM. doi: 10.1016/j.jdcr.2017.11.020.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5(1):54–56. From NLM. doi: 10.1016/j.jdcr.2018.10.010.
- Barroso-García B, Rial MJ, Molina A, et al. Alopecia areata in severe atopic dermatitis treated with dupilumab. J Investig Allergol Clin Immunol. 2018;28(6):420–421. From NLM. doi: 10.18176/jiaci.0301.
- Kanda N, Koto M, Hoashi T, et al. Case of alopecia areata during dupilumab treatment for atopic dermatitis. J Dermatol. 2019;46(9):e332–e333. From NLM. doi: 10.1111/1346-8138.14880.
- Yazdanyar S, Jemec GBE. Alopecia areata after treatment with dupilumab. Dermatitis. 2019;30(2):175–176. From NLM. doi: 10.1097/der.0000000000000458.
- Salgüero-Fernández I, Gonzalez de Domingo MA, Suarez D, et al. Dermatitis and alopecia in a patient treated with dupilumab: a new adverse effect? Clin Exp Dermatol. 2019;44(3):e41–e43. From NLM. doi: 10.1111/ced.13858.
- Chung J, Slaught CL, Simpson EL. Alopecia areata in 2 patients treated with dupilumab: new onset and worsening. JAAD Case Rep. 2019;5(8):643–645. From NLM. doi: 10.1016/j.jdcr.2019.03.019.
- Barbarin C, Hosteing S, Nosbaum A, et al. Early onset of alopecia areata after dupilumab introduction in a patient with atopic dermatitis. Eur J Dermatol. 2019;29(5):542–543. From NLM. doi: 10.1684/ejd.2019.3626.
- Carnicle JM, Hendricks AJ, Shi VY. Reactivation of alopecia areata after dupilumab therapy for atopic dermatitis. Dermatitis. 2021;32(1s):e80–e82. From NLM. doi: 10.1097/der.0000000000000512.
- Gallo R, Trave I, Parodi A. Massive acute alopecia of the scalp in a patient treated with dupilumab. Acta Derm Venereol. 2020;100(13):adv00191. From NLM. doi: 10.2340/00015555-3549.
- Ständer S, Trense Y, Thaçi D, et al. Alopecia areata development in atopic dermatitis patients treated with dupilumab. J Eur Acad Dermatol Venereol. 2020;34(10):e612–e613. From NLM. doi: 10.1111/jdv.16493.
- Maiolini VM, Sousa NA, Marsillac PF, et al. Alopecia areata-like and psoriasis after dupilumab use for atopic dermatitis. An Bras Dermatol. 2021;96(5):634–636. From NLM. doi: 10.1016/j.abd.2021.03.005.
- Beaziz J, Bouaziz JD, Jachiet M, et al. Dupilumab-induced psoriasis and alopecia areata: case report and review of the literature. Ann Dermatol Venereol. 2021;148(3):198–201. From NLM. doi: 10.1016/j.annder.2021.02.003.
- Yamane S, Nakagawa Y, Inui S, et al. Development of alopecia areata-like reactions in a patient treated with dupilumab. Allergol Int. 2022;71(3):420–422. From NLM. doi: 10.1016/j.alit.2022.02.006.
- Kulkarni M, Rohan CA, Morris D, et al. Resolution of dupilumab-associated alopecia areata with dosage modification. JAAD Case Rep. 2022;22:85–88. From NLM. doi: 10.1016/j.jdcr.2022.01.034.
- Jin P, Wei L, Zhang Q, et al. Dupilumab for alopecia areata treatment: a double-edged sword? J Cosmet Dermatol. 2022;21(11):5546–5548. From NLM. doi: 10.1111/jocd.15136.