Abstract
Aim: To evaluate the therapeutic efficacy and safety of JAK inhibitor abrocitinib in patients with localized granuloma annulare (GA) and to review the available cases documented in English.
Methods: We presented a patient who had a persistent, localized granuloma anulare (GA) for one year and did not respond to traditional therapies. This patient was treated with oral abrocitinib at a dosage of 150 mg daily.
Results: After 6 weeks of treatment with abrocitinib, the patient exhibited notable symptom improvement with no new lesions. No adverse events or recurrences were reported during the 5-month follow-up period.
Conclusions: Abrocitinib may be a promising and safe treatment option for patients with localized GA who do not respond to traditional therapies.
1. Introduction
Granuloma annulare (GA) is an inflammatory granulomatous skin disease that can be localized or generalized (Citation1). Patch, perforating, and subcutaneous subtypes are less common variants of this benign condition. Localized GA is often asymptomatic and self-limited within two years; however, patients may seek treatment for cosmetic reasons, with topical and intralesional corticosteroids being mainstays of therapy (Citation2). JAK inhibition has been reported to be effective in treating sarcoidosis—another inflammatory condition—and might also be effective in treating GA (Citation3,Citation4). This study details successful treatment using the Janus kinase (JAK) inhibitor abrocitinib in a patient with localized GA.
2. Case report
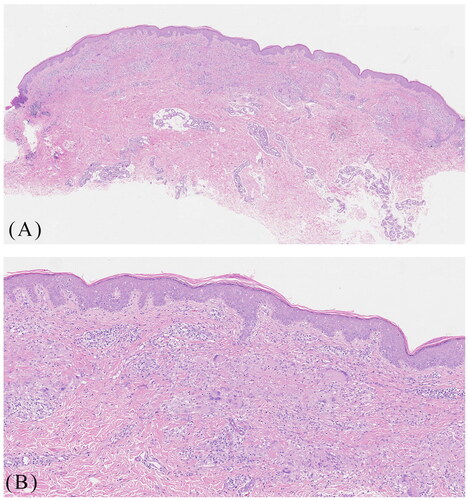
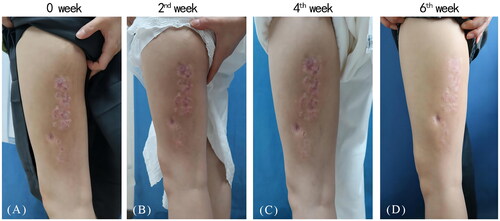
A 29-year-old female presented with one-year history of erythematous plaques and papules on her left lower extremity. The physical examination showed annular erythema with mild annular induration on the patient’s left lower extremity (). Histological examination revealed hyperkeratosis, epidermal thickening, and a palisaded arrangement of epithelioid granulomas containing lymphocytes, histiocytes, epithelioid cells, and multinucleated giant cells. Collagen degeneration was also observed between the granulomas (). The patient was diagnosed as GA and initiated traditional treatments including oral cyclosporine (50 mg twice daily) and hydroxychloroquine (0.2 g twice daily). However, significant improvement was not observed. Subsequently, we administered betamethasone injections into the patient’s skin lesions, which resulted in mild improvement but also caused mild hardening and atrophy of the lesions. The symptoms persisted and recurred. We then initiated a daily oral abrocitinib regimen at 150 mg. Surprisingly, within two weeks, the patient experienced thinning of the plaques, and after six weeks, substantial improvement was observed with no new lesions (). In the follow-up period of nearly five months, the patient remained satisfied with the control of her disease and no adverse events or recurrences were observed.
Figure 1. A 29-year-old female presented with annular erythema with mild annular induration on the left lower extremity before abrocitinib treatment (A), and progressive thinning of the plaques two, four weeks after abrocitinib treatment (B, C) and near complete clearance of lesions following six weeks of abrocitinib treatment (D).
3. Discussion
The upregulation of Th1, Th2, and JAK signaling has been associated with the expression of numerous inflammatory genes in GA (Citation5). IFN-γ, secreted by CD4+ T cells, triggers STAT1 activation in macrophages, which results in the production of IL-6. IL-6, in turn, activates STAT3 in T cells, which has been demonstrated to contribute to granuloma formation (Citation6). The JAK-STAT1 pathway in GA is partially activated due to increased activity of IFN-γ, oncostatin M (an IL-6 family cytokine), IL-15, and IL-21, as observed in GA (Citation4). Therefore, a potential therapeutic approach for GA involves blocking the JAK-STAT pathway by inhibiting these key cytokines. Abrocitinib is a JAK-1 selective inhibitor that has been FDA approved for moderate to severe atopic dermatitis. A recent study has revealed an association between GA and atopic diseases, which may be linked to Th2 pathway (IL-4/IL-13) deregulation (Citation7). This study is the first to report on the successful use of the JAK inhibitor abrocitinib in the treatment of GA. Recent case reports have described 4 GA patients who achieved complete symptom remission following eight months of oral tofacitinib treatment. Additionally, one patient treated with topical 2% tofacitinib achieved complete symptom resolution after 15 weeks (Citation8,Citation9). Furthermore, there is a report of a GA patient who achieved near-complete lesion clearance following four months of oral upadacitinib treatment (Citation10). Our report has limitations, including limited sample size and a relatively short treatment duration. Nevertheless, oral abrocitinib may be considered an effective treatment option for GA patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23(1):1–3. doi: 10.1007/s40257-021-00636-1.
- Thornsberry LA, English JC. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14(4):279–290. doi: 10.1007/s40257-013-0029-5.
- Slater KN, Valk B, Kartono F. A case of generalized granuloma annulare treated with upadacitinib. JAAD Case Rep. 2023;34:12–14. doi: 10.1016/j.jdcr.2023.01.027.
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147(5):1795–1809. doi: 10.1016/j.jaci.2020.10.012.
- Min MS, Wu J, He H, et al. Granuloma annulare skin profile shows activation of T-helper cell type 1, T-helper cell type 2, and Janus kinase pathways. J Am Acad Dermatol. 2020;83(1):63–70. doi: 10.1016/j.jaad.2019.12.028.
- Wang A, Singh K, Ibrahim W, et al. The promise of JAK inhibitors for treatment of sarcoidosis and other inflammatory disorders with macrophage activation: a review of the literature. Yale J Biol Med. 2020;93(1):187–195.
- Joshi TP, Chen V, Dong JL, et al. Atopic comorbidities associated with granuloma annulare: a case-control study of the all of us database. J Am Acad Dermatol. 2023;89(1):145–146. doi: 10.1016/j.jaad.2023.02.012.
- Bosch-Amate X, Serra-García L, Alamon-Reig F, et al. Treatment of granuloma annulare with tofacitinib. Australas J Dermatol. 2022;63(3):400–403. doi: 10.1111/ajd.13875.
- Damsky W, King BA. Treatment of granuloma annulare with tofacitinib 2% ointment. JAAD Case Rep. 2020;6(1):69–71. doi: 10.1016/j.jdcr.2019.10.016.
- Sondermann W, Hadaschik E, Specker C. Successful therapy of disseminated patch-type granuloma annulare with upadacitinib in a patient with rheumatoid arthritis. Dermatol Ther. 2022;35(1):e15211. doi: 10.1111/dth.15211.