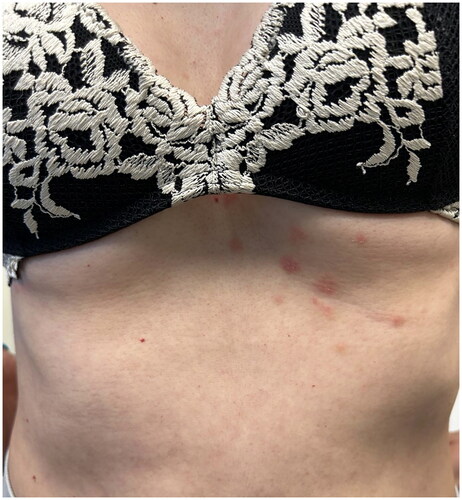
Dear Editor,
With the ongoing development and wide-scale use of different chemotherapy agents for oncologic diseases, several reports on cutaneous toxicities are emerging (Citation1). The onset of skin reactions represents a cause of treatment discontinuation in a significant percentage of oncological patients with a relevant impact on their prognosis (Citation1).
We report the case of a 33-year-old female hospitalized in the oncology division of our Institute to receive neoadjuvant chemotherapy for Ewing Sarcoma affecting the left tibia.
The treatment program was composed of nine cycles of vincristine + doxorubicin + cyclophosphamide (VDC scheme) alternated every 15 days with iphosphamide + etoposide (IE scheme) (Citation2).
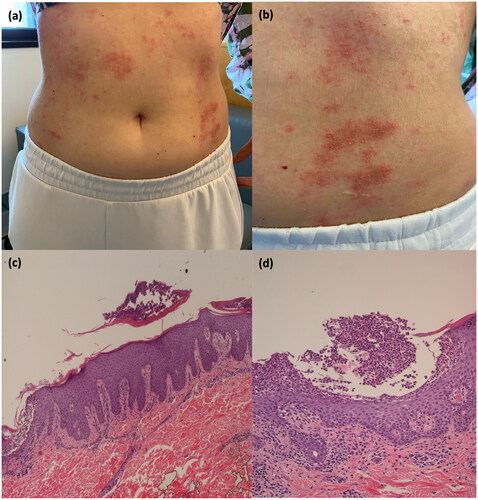
The patient was referred to our attention two weeks after the III cycle of IE infusion for the onset of a cutaneous rash. The patient reported a positive family history of psoriasis (mother). At physical examination, we observed erythematous plaques covered by multiple pin-point pustules spread to the trunk, upper and lower limbs (). The patient complained of intense itching and burning. We performed a 4.0 mm punch biopsy from an erythematous plaque on the back, and the histopathological examination showed epidermal hyperplasia with mild spongiosis, micro pustules and ortho-parakeratosis. Moreover, in the dermis, there was a scattered mixed inflammatory infiltrate with several polymorphonucleates ().
Figure 1. Erythematous plaques covered by multiple pustules in a 33-year-old female (). Histopathological picture of an incisional biopsy from a plaque on the back. Section of the skin characterized by epidermal hyperplasia, orto-parakeratosis, mixed inflammatory infiltrate in the dermis (), and micro pustules (). (c) Hematoxylin and Eosin stain, ×10 magnification; (d) Hematoxylin and Eosin stain, ×20 magnification
Since the patient reported a similar cutaneous reaction after the II cycle of IE, which resolved spontaneously, we decided, in accordance with the oncologist, not to administer the scheduled infusion.
Considering the time of onset, and the clinical and histological appearance of the manifestation, a diagnosis of pustular psoriasis was made, probably induced by the IE chemotherapy scheme in accordance with the Naranjo algorithm, which was calculated as 6 points (). Other potential diagnoses such as Steven-Johnson syndrome, DRESS (Drug reaction with eosinophilia and systemic symptoms), or toxic epidermal necrolysis were ruled out due to the lack of systemic symptoms, neutrophilia or eosinophilia, and mucosal lesions.
Table 1. Evaluation of causality relation according to Naranjo algorithm.
We prescribed a systemic therapy with iv methylprednisolone 40 mg, which was later switched to oral prednisone, starting with 25 mg with a progressive dosage reduction.
However, after steroid dose tapering, the patient had a relapse of the cutaneous rash. Thus, considering the patient’s cancer history and in agreement with the oncologists, we started therapy with apremilast according to the summary of product characteristics (Citation3). The patient did experience a rapid response, achieving a Psoriasis Area and Severity Index (PASI) ≤ 2 after the induction period (). To date, the patient is still on treatment with apremilast with no reported adverse events or relapses after a follow-up period of 6 months. The patient has completed the chemotherapy, and to date, she is undergoing clinical and instrumental follow-up, given the complete remission of the neoplasm.
Cutaneous toxicities emerging during non-targeted and targeted chemotherapies are common and have been widely described (Citation1,Citation4). Given the frequency and the impact of those adverse events on patients’ compliance with oncological treatments, several guidelines have been proposed to manage cutaneous toxicities related to these therapies (Citation4).
However, due to the low prevalence of Ewing Sarcoma, limited data on possible chemotherapy-related skin toxicities have been reported (Citation5,Citation6). Moreover, there is a paucity of data on cutaneous adverse reactions after iphosphamide or etoposide administration in these patients. Ilari et al. (Citation6) also described, among 26 patients receiving high-dose chemotherapy with etoposide, thiotepa and CY in treating poor-prognosis Ewing’s sarcoma family tumors, 20 cases of oral mucositis and 3 patients with skin toxicity (Citation6).
Apremilast, an oral phosphodiesterase-4 inhibitor, showed good effectiveness in patients with moderate-to-severe psoriasis also in those with the involvement of difficult-to-treat areas (Citation7,Citation8). Apremilast’s mechanism of action and safety profile make this drug a considerable option in patients with a history of chronic infections or patients with neoplasm. Thus, despite recent evidence confirming the safety of biologics also in this subset of patients, apremilast should be considered in the presence of chronic infections or oncological comorbidities (Citation9,Citation10).
We described pustular psoriasis emerging after IE infusion, which to the best of our knowledge, has not been reported yet. In our experience, apremilast was a successful treatment, confirming the encouraging safety and effectiveness data coming from both clinical trials and real-life experiences.
Ethical approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. The patient had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores) and for the publication of clinical pictures. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Disclosure statement
M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly and Boehringer Ingelheim. L. Gargiulo has been a consultant for Almirall. L. Ibba has been a consultant for Almirall. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. The other authors have nothing to disclose.
Data availability statement
Data available on request from the authors.
Additional information
Funding
References
- Shi VJ, Levy LL, Choi JN. Cutaneous manifestations of nontargeted and targeted chemotherapies. Semin Oncol. 2016;43(3):1–3. doi:10.1053/j.seminoncol.2016.02.018.
- Anderton J, Moroz V, Marec-Bérard P, et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours - EURO EWING 2012 protocol. Trials. 2020;21(1):96. Published 2020 Jan 17. doi:10.1186/s13063-019-4026-8.
- European Medicines Agency. Otezla (apremilast): summary of product characteristics. 2015 [cited 2023 December 6]. https://www.ema.europa.eu/en/medicines/human/EPAR/otezla.
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255–1268. doi:10.1016/j.jaad.2020.03.132.
- Brennan B, Kirton L, Marec-Bérard P, et al. Comparison of two chemotherapy regimens in patients with newly diagnosed Ewing sarcoma (EE2012): an open-label, randomised, phase 3 trial. Lancet. 2022;400(10362):1513–1521. Oct 29 doi:10.1016/S0140-6736(22)01790-1.
- Ilari I, De Ioris MA, Milano GM, et al. Toxicity of high-dose chemotherapy with etoposide, thiotepa and CY in treating poor-prognosis Ewing’s sarcoma family tumors: the experience of the Bambino Gesù Children’s Hospital. Bone Marrow Transplant. 2010;45(8):1274–1280. doi:10.1038/bmt.2009.353.
- Pavia G, Gargiulo L, Cortese A, et al. Apremilast for the treatment of palmo-plantar non-pustular psoriasis: a real-life single-center retrospective study. Dermatol Ther. 2022;35(2):e15253. Feb doi:10.1111/dth.15253.
- Ständer S, Syring F, Ludwig RJ, et al. Successful treatment of refractory palmoplantar pustular psoriasis with apremilast: a case series. Front Med (Lausanne). 2020;7:543944. doi:10.3389/fmed.2020.543944.
- Valenti M, Pavia G, Gargiulo L, et al. Biologic therapies for plaque type psoriasis in patients with previous malignant cancer: long-term safety in a single- center real-life population. J Dermatolog Treat. 2022;33(3):1638–1642. doi:10.1080/09546634.2021.1886231.
- Gargiulo L, Pavia G, Valenti M, et al. Safety of biologic therapies in patients with moderate-to-severe plaque psoriasis and concomitant viral hepatitis: a monocentric retrospective study. Dermatol Ther (Heidelb). 2022;12(5):1263–1270. doi:10.1007/s13555-022-00726-w.