Abstract
Background
As one of the most effective biologic treatments for psoriasis, the short-term effectiveness of ustekinumab has yet to be studied extensively.
Objective
The purpose of this study was to evaluate the short-term effectiveness and potential factors within four weeks after the first-dose ustekinumab treatment based on real-world data.
Methods
The study enrolled 98 patients with moderate-to-severe psoriasis, given ustekinumab 45 mg at week 0, week 4, and then every 12 weeks. Based on clinical data collected at baseline and week 4, we investigated the short-term effectiveness of ustekinumab after the first dose and potential factors associated with the treatment. For evaluation, we collected demographic information, body data, medical history, laboratory examination results, Psoriasis Area and Severity Index (PASI), body surface area (BSA), and dermatology life quality index (DLQI). Response rates were calculated based on the number of patients that achieved a 75/90/100% reduction in PASI (PASI 75/90/100), and the primary treatment goal was to achieve PASI 75.
Results
The response rates for PASI 75/90/100 at week 4 were 30.5%, 18.9%, and 16.8%, respectively. For PASI 75, the response rate was higher in patients without metabolic syndrome (MS) (without MS vs. with MS: 36.9% vs. 5.9%, p = 0.013); the serum triglyceride (TG) level was significantly lower in patients achieving PASI 75 (expressed as mean ± standard deviation, achieved vs. unachieved: 1.82 ± 1.79 vs. 3.59 ± 8.89, p = 0.010). For PASI 100, the response rates were higher in female patients (female vs. male: 26.3% vs. 10.5%, p = 0.044) and patients with a family history of psoriasis (with family history vs. without family history: 44.4% vs. 13.9%, p = 0.042). In addition, the possibility of achieving PASI 75/90/100 went up along with the serum high-density lipoprotein cholesterol (HDL-C) level (expressed as adjusted odds ratio < 95% confidence interval>: PASI 75: 28.484 < 2.035-248.419>, p = 0.011; PASI 90: 28.226 < 2.828-281.729>, p = 0.004; PASI 100: 12.175 < 1.876-79.028>, p = 0.009).
Conclusion
In this study, nearly one-third of patients achieved PASI 75 after only the first-dose ustekinumab treatment. Sex, family history of psoriasis, MS, serum TG level might affect the short-term effectiveness, and serum HDL-C level may be a potential factor. The possibility of achieving treatment goals (PASI 75/90/100) at week 4 increased along with serum HDL-C levels.
Introduction
Psoriasis is a chronic immune-mediated papulosquamous skin disease consisting of systemic manifestations (Citation1), with a prevalence being 0.51%-11.43% of the worldwide population (Citation2,Citation3). Various comorbidities could be accompanied with psoriasis, including metabolic syndrome (MS), which includes central obesity, hyperglycemia or diabetes mellitus, dyslipidemia, and hypertension (Citation4–6), and is associated with increased disease severity of psoriasis, impaired biologic therapeutic response, and the decrease of effectiveness persistence (Citation6–9).
Biologics that inhibit specific target cytokines, such as interleukin (IL)-12, IL-17, IL-23, and tumor necrosis factor-α (TNF-α) (Citation9), have become popular and effective treatment choices. Ustekinumab (Stelara, Janssen Biotech) is specifically blocking the p40 subunit in IL-23(Citation10, Citation11), inhibiting the inflammatory cascade amplification, diminishing primary signals to pathologically drive the development of psoriasis (Citation12,Citation13). Compared with other biologics approved in the medicine market, ustekinumab requires less frequency and dosage, with a relatively long interval between administrations (Citation14). Thus, commonly it is the long-term rather than the short-term efficacy that has been focused more in randomized clinical trials (RCTs) and observational clinical trials (Citation15–20). More rarely, so far, there has not been much research focusing on the effects that MS causes on short-term efficacy.
This study aims to collect real-world data after the first-dose treatment of ustekinumab among Chinese patients to analyze the short-term effectiveness. Also, we intend to investigate whether the factors related to MS or other clinical characteristics may affect short-term effectiveness.
Methods
Patients and materials
This study enrolled moderate-to-severe psoriasis patients consecutively at the Department of Dermatology in Xiangya Hospital of Central South University from January to October 2022. Patients were required to accept the first dose and follow-up treatments at our hospital except for special conditions. The washout period was not required between medication switches unless the reason was adverse events appearance (Citation21,Citation22), in which case the washout period for previously used systematic drugs ranged from 1 month to 3 months (3–4 half-life periods of the former drug). A series of premedical examinations were done for every patient to ensure no contraindication for ustekinumab. The routine treatment dosage of ustekinumab is 45 mg (patients with weight <100 kg) or 90 mg (patients with weight ≥100 kg) every time; the frequency of administration is week 0, week 4, and then every 12 weeks (Q12W) (Citation17,Citation18).
Ustekinumab is applicable to adult patients with moderate-to-severe plaque psoriasis, in the condition of having contraindications or intolerance to or failing to respond to cyclosporine, methotrexate (MTX) or psoralen and ultraviolet A (PUVA) and other systematic treatments (Citation23). All patients were informed of the potential risks, theoretical benefits, and possibilities of ineffectiveness.
Patients were diagnosed by experienced dermatologists that were experts in psoriasis. Except for diagnosis, other clinical data collected consisted of demographic characteristics (age and sex) and clinical characteristics. The latter one consisted of body data (height, weight, waist circumstance, and blood pressure), medical history, laboratory examination results, Psoriasis Area and Severity Index (PASI, scores range from 0 to 72 with higher scores indicating greater disease severity), body surface area (BSA), and dermatology life quality index (DLQI, scores range from 0 to 30 with higher scores indicating more significant effects on daily life). Besides, the family history of psoriasis was assessed among first-degree and second-degree relatives. The laboratory examination results included records of fasting serum lipids and fasting serum glucose. Metabolic syndrome was defined according to the Chinese guidelines, meeting at least three of the following criteria: (1) abdominal obesity(elevated waist circumference: ≥90 cm in males or ≥85 cm in females); (2) elevated fasting blood glucose(≥6.1 mmol/L) or medical treatment for hyperglycemia; (3) elevated blood pressure(systolic/diastolic blood pressure ≥ 130/85 mmHg) or medical treatment for a history of hypertension; (4) elevated fasting triglyceride (≥1.7 mmol/L); and (5) low fasting high-density lipoprotein cholesterol (HDL-C, <1.0 mmol/L) (Citation24).
PASI, BSA, and DLQI were recorded to reflect disease severity. Follow-up visits were completed after the first-dose treatment at week 4 before the second dose injected. Adverse events (AEs) and treatment discontinuation reasons were also recorded. Evaluations mentioned above were assessed blindly by experienced dermatologists to ensure reliability. The response rates were calculated based on the number of patients that achieved a ≥ 75/90/100% reduction in PASI (PASI 75/90/100), and the primary treatment goal was to achieve PASI 75. Effectiveness evaluations were only analyzed among data that could be observed, while patients not eligible were not included.
This study was conducted by the principles of the Declaration of Helsinki and approved by the Ethics Committee of Xiangya Hospital of Central South University (approval number: 2018121106). Before participation, all the adult patients have provided written informed consent confirmed by themselves; patients under 18 also have provided written informed consent approved by their parents or legal guardians.
Statistical analyses
Descriptive statistics were calculated for continuous quantitative variables and expressed as mean ± standard deviation (mean ± SD). For categorical variables, frequencies were calculated and defined as frequency(percentages) (n<%>). Considering the relatively small data quantity, the Shapiro-Wilk test was used for the normality distribution test. T-test and Mann-Whitney U-test were conducted to analyze statistical differences for continuous variables meeting normal or non-normal distribution. The Chi-Square test was used for statistical difference and stratified analysis of response rates for categorical variables. The Fisher’s precision probability test was used when the Chi-Square test was not applicable (the sample size n < 40 or the expected frequency T < 5). Bivariate logistic regression was conducted among factors significantly different between groups divided by the achievement of effectiveness indicators to reflect the association. All statistical analyses were accomplished in SPSS 26.0, and charts were drawn in GraphPad Prism 9.3. p < 0.05 was considered statistically significant.
Results
Baseline characteristics
The demographic and clinical characteristics at baseline of all patients enrolled are summarized in . Our study recruited 98 patients receiving at least one ustekinumab treatment ().
Table 1. Baseline characteristics of all the patients included.
The average age of all the patients included is 42 (42.0 ± 14.0) years old. The average onset age is 31 (30.8 ± 14.8) years old, with an average disease duration of 11 (11.2 ± 9.5) years. One-tenth of the patients (9/90) had a family history of psoriasis. As for the history of other medication administration, all of the patients had received topical drugs before, 7 patients had previously received other biologics (2 of adalimumab, 3 of secukinumab, and 2 of ixekizumab), which all aim at other target cytokines (TNF-α and IL-17). Reasons for changing to ustekinumab treatment included ineffectiveness (n = 6) and adverse events (n = 1, eczema). Among 83 patients with laboratory examination results preserved in our medical record system, 17(20.5%) patients were accompanied by MS at baseline. The average values (expressed as mean ± SD) of PASI, BSA, and DLQI at baseline were 9.4 ± 4.8, 11.2 ± 8.7 and 7.4 ± 5.0 respectively.
Effectiveness
To reveal the effectiveness both on the disease severity and the quality of life, we summarized the numerical variations in PASI, BSA, and DLQI. All patients enrolled were included in the effectiveness analyses. Notably, all 98 patients had completed the evaluation at baseline. In comparison, 3 patients failed to come to our hospital in person to meet the follow-up at week 4 (2 patients due to the local epidemic prevention and control policies, and 1 patient due to discontinuation caused by transaminase increasing).
The changes in PASI and BSA reflected the skin lesion severity. The mean value of PASI decreased from 9.4 at baseline to 3.7 at week 4, with the average PASI reduction of 5.9 after the first dose over 4 weeks, showing an apparent downward trend (). The percentages of average PASI reduction (compared to baseline) were 60.1%. The response rate of patients achieving the primary goal (PASI 75) after the first dose was 30.5%. The percentages of patients achieving PASI 90/100 at week 4 were 18.9% and 16.8%, respectively (). Meanwhile, the average value of BSA declined from 11.2 at baseline to 4.8 at week 4, with an average reduction of 4.6 ().
Table 2. Efficacy responses at week 4 after single-dose treatment.
From the perspective of changes in quality of life, the mean value of DLQI at baseline and week 4 were 7.6 and 2.7, respectively (), with an average reduction of 4.9, also showing an apparent downward trend (). Besides, the percentages of patients achieving DLQI 0/1 were raised from 9.2% at baseline to 46.3% at week 4.
Factors associated with medication responses
We conducted univariate analyses to screen potential factors that may be associated with short-term effectiveness. For the primary treatment goal (PASI 75), the results indicated that the response rate was higher in patients without MS (without MS vs. with MS: 36.9% vs. 5.9%, p = 0.013) (). Also, the serum TG level was significantly lower in patients achieving the primary treatment goal (mean ± SD, PASI 75 achieved vs. PASI 75 unachieved: 1.82 ± 1.79 vs. 3.59 ± 8.89, p = 0.010) (). For the skin lesion clearance (PASI 100), the response rates were higher in female patients (female vs. male: 26.3% vs. 10.5%, p = 0.044) () and patients with a family history of psoriasis (with family history vs. without family history: 44.4% vs. 13.9%, p = 0.042) (). Notably, the serum HDL-C levels were significantly higher in patients achieving PASI 75/90/100 respectively (mean ± SD, achieved vs. unachieved: PASI 75: 1.48 ± 0.47 vs. 1.17 ± 0.24, p < 0.001; PASI 90: 1.57 ± 0.55 vs. 1.09 ± 0.25, p < 0.001; PASI 100: 1.57 ± 0.58 vs. 1.20 ± 0.26, p = 0.002). Therefore, we conducted further analyses to aid the clearness of the association between the potential factors and response rates.
Figure 1. Proportion of patients achieving PASI 75 at week 4 in groups with or without metabolic syndrome. With metabolic syndrome vs. metabolic syndrome: 5.9% vs. 36.9%, p = 0.010. PASI 75: ≥75% reduction in Psoriasis Area and Severity Index.
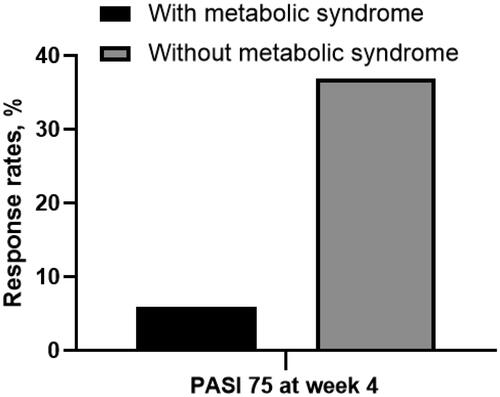
Figure 2. Proportion of patients achieving PASI 75 at week 4 in male or female patients. Male vs. female: 10.5% vs. 26.3%, p = 0.047. (PASI 100: 100% reduction in Psoriasis Area and Severity Index).
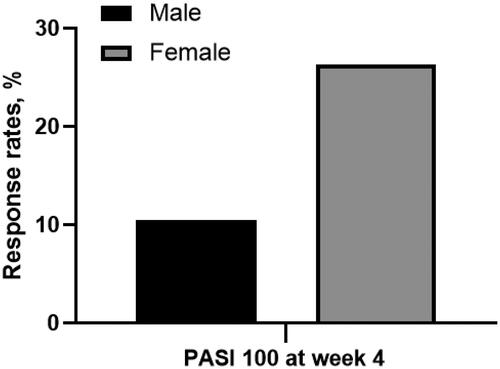
Figure 3. Proportion of patients achieving PASI 75 at week 4 in groups with or without family history of psoriasis. With family history vs. without family history: 44.4% vs. 13.9%, p = 0.044. (PASI 100: 100% reduction in Psoriasis Area and Severity Index).
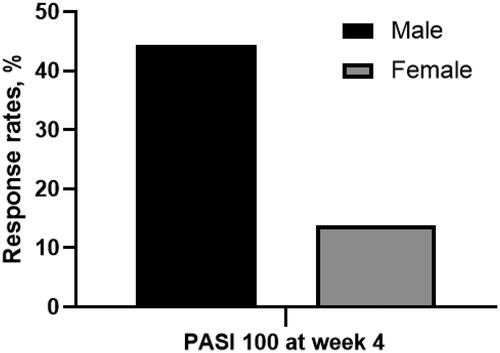
Table 3. Results of difference analysis between groups whether achieving PASI 75/90/100 at week 4.
Next, we conducted the binary logistic regression between potential factors and medication effectiveness based on univariate analysis results. With consideration of sex, age, and disease duration being correction factors, the results suggested that the possibility of achieving different treatment goals (PASI 75/90/100) after the first dose of ustekinumab went up along with the value of serum HDL-C level arose (expressed as adjusted odds ratio < 95% confidence interval>: PASI 75: 28.484 < 2.035-248.419>, p = 0.011; PASI 90: 28.226 < 2.828-281.729>, p = 0.004; PASI 100: 12.175 < 1.876-79.028>, p = 0.009) (.
Table 4. Association between PASI 75 /90/100 response and baseline characteristics.
Safety
A total of 3(0.3%) patients reported adverse events (AEs) during the 4-week observation, which were skin pruritus (n = 1), elevated transaminase (n = 1) and eczema (n = 1) (). The patient with elevated transaminase tested at local hospital after the first-dose ustekinumab treatment was the only case leading to drug discontinuation in this study. No severe adverse event (SAE) was observed during this study.
Table 5. Adverse events during the 4-week treatment period.
Discussion
MS has been reported to affect the long-term medication response of biologic therapy (Citation9). However, few researches have validated the effect of MS on the short-term effectiveness of ustekinumab yet. Throughout this study, we aimed to observe and analyze real-world data on the short-term effectiveness of ustekinumab after the first dose. Firstly, within the four-week observation period, 30.5% of patients achieved the primary treatment goal (PASI 75) after receiving only the first-dose ustekinumab treatment (). Secondly, we found that sex, family history of psoriasis, MS, serum TG level, and serum HDL-C level were possible factors of short-term effectiveness ( and Citation4). Further, when adjusted by sex, age, and disease duration, the possibility of achieving treatment goals (PASI 75/90/100) at week 4 increased along with serum HDL-C level arose.
Even though the confidence interval of HDL-C ratio is wide as mentioned above, which could be related to various reasons like the small sample size, the relatively short observation period, and ethnic factor, we still considered serum HDL-C level a potential predictor for the short-term effectiveness. Interestingly, Chau Yee Ng et al. found that after the 24-week observation period, the serum HDL-C level remained unchanged (Citation25). The serum HDL-C level is susceptible to various factors, such as lifestyle, liver function, genetic factor et al. thus showing fluctuations (Citation25–27). This present study found significant serum HDL-C level changes in the short-term, which might be a temporary phenomenon. Not only does HDL-C play a decisive role in the cholesterol reverse transportation, but it is also important in anti-inflammation process (Citation28). Thus, from a long-term perspective, when the systemic utilization of adipose tissue tends to balance and the inflammation diminishes, the impact of ustekinumab on HDL may tend to flatten out, as the results shown in another 52-week observational study (Citation29,Citation30). Future studies might be necessary to find predicting factors responsible for metabolic parameter changes in Chinese psoriasis patients treated with ustekinumab.
In a network meta-analysis of various IL-12/23 biologics for the treatment of psoriasis, ustekinumab performed mediocre in terms of efficacy and tolerability (Citation23). However, in this 4-week observational study, achievements were obtained both in skin lesion clearing and quality-of-life improvement. For skin lesion clearing, compared with the RCT data (Citation31), the real-world data in this study showed more effective results in the medication response rates after the first-dose application (real-world data vs. RCT data: PASI 75: 30.5% vs. 20.6%; PASI 90: 18.9% vs. 5.4%; PASI 100: 16.8% vs. 0.9%). Nearly one-third of the patients in this study achieved the primary treatment goal (PASI 75) after only the first-dose ustekinumab treatment. And with the obvious reduction in PASI scores, 96.9% (95/98) of the patients chose to continue the subsequent treatments. Next, as for the quality of life, the mean value of DLQI dropped from 7.6 to 2.7 during the 4-week observation. In addition, DLQI 0/1 could be considered as having no effect on daily life (Citation32). In this study, the percentage of patients achieving DLQI 1/0 increased nearly tenfold before and after the first-dose application (from 9.2% to 42.3%), which indicated that nearly half of the patients cast off the extra burden on daily life in the short-term. The achievement of the primary treatment goal and the improvement of the quality of life in the short-term may assist to raise the confidence and compliance of patients.
As regards factors associated with the short-term response, data in our research found that the response rates were higher in females and patients with a family history of psoriasis. Some studies have suggested that female gender may be associated with a decreased response to biologic therapies (Citation33, Citation34). However, in an eight-year study on ustekinumab treatment in psoriasis, real-life experience results showed better effectiveness in female gender (Citation35). Moreover, Delia et al.’s research findings indicated that there was no obvious correlation between gender and effectiveness of biologic therapies (Citation36). Moreover, Therefore, whether there is a significant correlation between gender and biologic therapy effectiveness still remains unclear. Reasons for these different results may be attributed to racial composition diversity and variations in geographical environments. Therefore, it may be the environmental and ethnic differences contributing to higher medication response in Chinese females of skin lesion clearance (PASI 100) in this study (female vs. male: 26.3% vs. 10.5%, p = 0.044). Also, regardless of the degrees of consanguinity therein, patients with a family history of psoriasis were more likely to achieve skin lesion clearance after the first-dose application (with family history vs. without family history: 44.4% vs. 13.9%, p = 0.042). It is validated that family history may be a risk factor for developing psoriasis (Citation37–39), but temporarily few studies focused on the impact on medication response obtaining or maintaining, in which case further large-scale researches were needed.
In addition, metabolic parameters including MS, serum TG level, and serum HDL-C level were also displayed to affect the short-term effectiveness The appearance of MS and dyslipidemia are related to impaired pro-inflammatory cytokines, glucose metabolism, and vascular endothelial biology (Citation40,Citation41). The abnormal lipid accumulation and impaired pharmacokinetic factors then caused chronic systemic inflammation, metabolic disorders, and impaired therapeutic response (Citation42,Citation43). However, by promoting cholesterol reverse transportation from macrophages (Citation44), the special serum lipid parameter, HDL-C, is inversely associated with the risk of lipid dysregulation (Citation45), which might help to explain the result that higher serum HDL level could improve and increase the possibility to achieve short-term effectiveness in this study. Consequently, putting the medical popular science education for psoriasis patients on a healthy lifestyle may assist to avoid developing MS and keep serum TG and HDL-C levels in the normal range. It may subsequently help to acquire better medication responses and raise their compliance with the subsequent treatments.
Our study has several potential limitations that may affect the interpretation of our findings. Firstly, although we focused on the short-term effectiveness of ustekinumab, the observation period of our study was only 4 weeks, which may not completely rule out the possibility that some patients who did not achieve response in the short term could benefit from ustekinumab treatment in the long term. Secondly, the sample size of the study was relatively small, which may introduce bias in the results, and the lack of comparison with other treatment modalities limits a comprehensive evaluation of the advantages and disadvantages of ustekinumab in the treatment of psoriasis. Lastly, the study population consisted exclusively of individuals of Chinese ethnicity, therefore the generalizability of the findings to the wider global population may be limited.
Conclusion
Nearly one-third of patients achieved the primary treatment goal (PASI 75) after only the first-dose ustekinumab treatment. Sex, family history of psoriasis, metabolic syndrome, serum TG level, and serum HDL-C level may be associated with the short-term effectiveness. Furthermore, when adjusted by sex, age, and disease duration, the possibility to achieve different treatment goals (PASI 75/90/100) at week 4 went up along with the value of serum HDL-C level arose in this study, making serum HDL-C level a potential factor.
Authors’ contribution
Xingyu Li and Xiaowen Xie performed the study design, data analysis, and manuscript writing. Jiashuai Li, Jingjin Hu, Minjia Tan, Jing Yang, Sichun Deng, and Yijie Liu contributed to the data collection and validation. Mi Zhang, Junchen Chen, Liqiu Liao, Yehong Kuang, and Wu Zhu performed the diagnosis, sample collections, and/or manuscript revising. Yehong Kuang and Wu Zhu were clinic experts and gave suggestions for manuscript revision. All authors read and approved the final version of the manuscript.
Statement of ethics
The principles outlined in the Declaration of Helsinki were followed in our research. This study was reviewed and approved by the institutional research ethics boards of Xiangya Hospital (approval number: 2018121106). Written informed consent was obtained from all of the adult participants and from parents/legal guardians of all participants under 18 years old.
Acknowledgment
The authors would like to extend their sincerest appreciation to all coordinators, dermatologists, and investigators who participated in this study.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability Statement
All data generated or analyzed during this research are included in this article. Further requires can be directed to the corresponding authors.
Additional information
Funding
References
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1–8. doi:10.1016/S0140-6736(20)32549-6.
- Ghoreschi K, Balato A, Enerbäck C, et al. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi:10.1016/S0140-6736(21)00184-7.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi:10.1111/jdv.13854.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi:10.1001/jamadermatol.2013.5015.
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. Jama. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006.
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77(4):657–666.e8. doi:10.1016/j.jaad.2017.04.1133.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–562. doi:10.1038/jid.2011.365.
- Liu L, Cai XC, Sun XY, et al. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: current evidence. J Eur Acad Dermatol Venereol. 2022;36(11):1969–1979. doi:10.1111/jdv.18296.
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84(2):398–407. doi:10.1016/j.jaad.2020.09.047.
- Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3(6):535–545. doi:10.4161/mabs.3.6.17815.
- Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–1214. doi:10.1056/NEJMoa1900750.
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133(12):2741–2752. doi:10.1038/jid.2013.237.
- Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. doi:10.1016/j.jaci.2017.07.004.
- Girolomoni GSR, Puig L, Bachelez H, et al. The role of IL-23 and the IL-23/T(H)17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(10):1616–1626. doi:10.1111/jdv.14433.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–592. doi:10.1056/NEJMoa062382.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–252. doi:10.1111/j.1346-8138.2011.01347.x.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–1674. doi:10.1016/S0140-6736(08)60725-4.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–1684. doi:10.1016/S0140-6736(08)60726-6.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in taiwanese and korean patients (PEARL). J Dermatol Sci. 2011;63(3):154–163. doi:10.1016/j.jdermsci.2011.05.005.
- Zhu X, Zheng M, Song M, et al. Efficacy and safety of ustekinumab in chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol. 2013;12(2):166–174.
- Mrowietz U, de Jong EM, Kragballe K, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28(4):438–453. doi:10.1111/jdv.12118.
- Tsai YC, Tsai TF. Switching biologics in psoriasis - practical guidance and evidence to support. Expert Rev Clin Pharmacol. 2020;13(5):493–503. doi:10.1080/17512433.2020.1767590.
- Bai F, Li GG, Liu Q, et al. Short-Term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network Meta-Analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161–2546125. doi:10.1155/2019/2546161.
- Jun-Ren ZHU, Run-Lin GAO, Shui-Ping ZHAO, et al. Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15(1):1–29.
- Ng CY, Huang Y-H, Tzeng I-S, et al. Changes in metabolic parameters in psoriasis patients treated with interleukin-12/23 blockade (ustekinumab). Dermatol Sin. 2020;38(3):166–171. doi:10.4103/ds.ds_27_20.
- Escolà-Gil JC, Julve J, Griffin BA, et al. HDL and lifestyle interventions. High Density Lipoproteins: from Biological Understanding to Clinical Exploitation. 2015;224:569–592. doi:10.1007/978-3-319-09665-0_18.
- Nikkilä EA, Taskinen M-R, Kekki M. Relation of plasma high-density lipoprotein cholesterol to lipoprotein-lipase activity in adipose tissue and skeletal muscle of man. Atherosclerosis. 1978;29(4):497–501. doi:10.1016/0021-9150(78)90178-8.
- Peter Wolf M, Wolfgang Weger M, Thomas O, et al. Biological anti-psoriatic therapy profoundly affects high-density lipoprotein function.
- Hagino T, Saeki H, Fujimoto E, et al. Effects of biologic therapy on laboratory indicators of cardiometabolic diseases in patients with psoriasis. J Clin Med. 2023;12(5):1934. doi:10.3390/jcm12051934.
- Roig CA, Ferrer CS, García JLR, et al. Influence of biologic therapy on cardiovascular risk factors in patients with inflammatory bowel disease. Gastroenterología y Hepatología (English Edition). 2023;46(2):109–115. doi:10.1016/j.gastre.2022.05.003.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi:10.1016/j.jaad.2015.05.013.
- Papp KA, Lebwohl MG, Puig L, et al. Long-term efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial beyond 3 years of follow-up. Br J Dermatol. 2021;185(6):1135–1145. doi:10.1111/bjd.20595.
- Gonulal M, Balci DD, Ozturk A, et al. Effectiveness and safety of ustekinumab for the treatment of psoriasis; six years of clinical experience. J Dermatolog Treat. 2023;34(1):2241941.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to anti-tumor necrosis factor therapies in patients with moderate to severe psoriasis: real-world data from the US corrona psoriasis registry. J Dermatolog Treat. 2021;32(3):302–309. doi:10.1080/09546634.2019.1656797.
- Galluzzo M, D’Adamio S, Silvaggio D, et al. Ustekinumab treatment for moderate-to-severe plaque psoriasis: eight-year real-life experience. Expert Opin Biol Ther. 2020;20(1):95–104. doi:10.1080/14712598.2020.1684472.
- Colombo D, Bianchi L, Fabbrocini G, et al. The CANOVA study real-world evidence of biologic treatments in moderate-severe psoriasis in Italy: a gender perspective. Womens Health Rep (New Rochelle). 2022;3(1):450–457. doi:10.1089/whr.2021.0124.
- Ya J, Hu JZ, Nowacki AS, et al. Family history of psoriasis, psychological stressors, and tobacco use are associated with the development of tumor necrosis factor-α inhibitor-induced psoriasis: a case-control study. J Am Acad Dermatol. 2020;83(6):1599–1605. doi:10.1016/j.jaad.2020.06.081.
- Egeberg A, Griffiths CEM, Williams HC, et al. Clinical characteristics, symptoms and burden of psoriasis and atopic dermatitis in adults. Br J Dermatol. 2020;183(1):128–138. doi:10.1111/bjd.18622.
- Solmaz D, Bakirci S, Kimyon G, et al. Impact of having family history of psoriasis or psoriatic arthritis on psoriatic disease. Arthritis Care Res (Hoboken). 2020;72(1):63–68. doi:10.1002/acr.23836.
- Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf). 2006;64(4):355–365. doi:10.1111/j.1365-2265.2006.02474.x.
- Shirai K. Obesity as the core of the metabolic syndrome and the management of coronary heart disease. Curr Med Res Opin. 2004;20(3):295–304. doi:10.1185/030079903125003008.
- Hamminga EA, van der Lely AJ, Neumann HA, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67(4):768–773. doi:10.1016/j.mehy.2005.11.050.
- Carrascosa JM, Rocamora V, Fernandez-Torres RM, et al. Obesity and psoriasis: inflammatory nature of obesity, relationship between psoriasis and obesity, and therapeutic implications. Actas Dermosifiliogr. 2014;105(1):31–44. doi:10.1016/j.ad.2012.08.003.
- Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. doi:10.1056/NEJMoa1001689.
- Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi:10.1161/01.cir.79.1.8.

