To the Editor,
We read with interest the recently published article by De La Torre-Gomar et al. dealing with a patient affected by atopic dermatitis and extensive alopecia areata (AA) responsive to upadacitinib following a relapse of both disorders after six months of baricitinib treatment (Citation1). We also experienced relapses in three patients with severe AA undergoing treatment with baricitinib, a Janus Kinase (JAK) 1–2 inhibitor recently approved both in the United States by the Food and Drug Administration and in Europe by the European Medicine Agency, for the treatment of adults with severe AA (Citation2,Citation3).
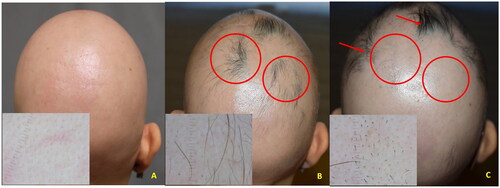
Case 1: a 28-year-old woman with a history of AA universalis since 5 years of age with transient response to different AA treatments, including intralesional corticosteroids, contact immunotherapy with squaric acid dibutylester (SADBE), cyclosporine, and methotrexate. The patient was treated with baricitinib 4 mg once daily, resulting in initial hair regrowth after four weeks of treatment. However, at week 20 (SALT = 75), a bilateral relapse occurred within the occipital and parietal areas of regrowth (). Laboratory investigations were unremarkable, showing only mild anemia. Trichoscopy, showing black dots and exclamation mark hairs, and histopathology confirmed the diagnosis of active AA.
Figure 1. A (baseline): clinical aspect and dermoscopy (insert) of AA universalis; B (three months): regrowth (circles) under baricitinib treatment (4 mg/day); C (five months): relapse within the regrowth areas (circles) while under baricitinib (Phenotype 1); localized new areas of regrowth were observed (arrows).
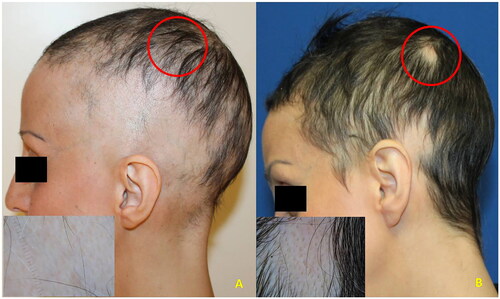
Case 2: a 43-year-old woman with severe AA (SALT = 70) occurred 2 years earlier. Previous treatments with intralesional and systemic corticosteroids, oral cyclosporine, and contact immunotherapy with SADBE had been ineffective. Baricitinib treatment was started and at week 4 progressive hair regrowth was observed. At week 12 (SALT = 10) the patient developed a new AA patch on the previously unaffected left parieto-temporal area (). At trichoscopy, multiple black dots and exclamation mark hairs were evident.
Figure 2. A (baseline): clinical aspect and dermoscopy (insert) of severe AA (SALT = 70); B (three months): relapse within a previously unaffected area (circle) while under baricitinib treatment (4 mg/day) (Phenotype 2).
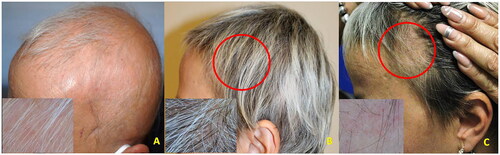
Case 3: a 52-year-old woman with severe AA (SALT = 90) occurred 2 years earlier unresponsive to previous treatments with systemic corticosteroids and methotrexate. Baricitinib treatment was started and at week 4 a progressive hair regrowth was observed. At week 24 (SALT = 5), a relapse occurred bilaterally within the frontotemporal regrowth areas (). At trichoscopy multiple black dots and exclamation mark hairs were evident. Histology confirmed the diagnosis of active AA.
Figure 3. A (baseline): clinical aspect and dermoscopy (insert) of severe AA (SALT = 90); B (five months): regrowth under baricitinib treatment (4 mg/day); C (six months): relapse within the regrowth area (circle) while under baricitinib (Phenotype 1).
None of our patients had a personal or family history of atopy or atopic dermatitis. Treatment (4 mg/day) was not discontinued in any of them and all are under strict follow-up.
Our observations confirm that AA relapses may occur during baricitinib treatment (Citation1–3). Interestingly, hair loss developed either within the regrowth area as seen in Cases 1 and 3 or in a healthy area previously unaffected as in Case 2, confirming two different presentation phenotypes.
We believe that during pretreatment counseling with the patient undergoing baricitinib treatment for AA it is essential to discuss not only about the expected therapeutic response and the potential adverse events, but also of the possible occurrence of relapses. These can occur after discontinuation of therapy, as previously observed (Citation1), or during treatment with the presented different phenotypes. The long-term clinical response of patients with AA with baricitinib is warranted.
Ethics statement
Appropriate internal hospital IRB approval (Comitato Etico Catania 1, A.O.U. Policlinico G. Rodolico-San Marco) has been obtained before initiation of treatment. Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- De la Torre-Gomar FJ, Velasco-Amador JP, Prados-Carmona Á, et al. Complete response of extensive alopecia areata refractory to baricitinib after five months of treatment with upadacitinib. J Dermatolog Treat. 2024;35(1):1. doi: 10.1080/09546634.2024.2304630.
- Kwon O, Senna MM, Sinclair R, et al. Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol. 2023;24(3):443–2. doi: 10.1007/s40257-023-00764-w.
- Yan D, Fan H, Chen M, et al. The efficacy and safety of JAK inhibitors for alopecia areata: a systematic review and meta-analysis of prospective studies. Front Pharmacol. 2022;13:950450. doi: 10.3389/fphar.2022.950450.

