Abstract
Background
Lebrikizumab improved itch, interference of itch on sleep, and quality of life (QoL) in patients with moderate-to-severe atopic dermatitis (AD), in two Phase 3 trials at 16 weeks compared to placebo.
Objectives
We assess improvements in itch and sleep interference due to itch and their impact on QoL measurements after treatment.
Methods
Data were analyzed from ADvocate1 (NCT04146363) and ADvocate2 (NCT04178967) in patients with moderate-to-severe AD. QoL was evaluated using Dermatology Life Quality Index (DLQI) at Week 16 in patients (>16 years of age) who were itch responders/non-responders (defined as ≥4-point improvement in Pruritus Numeric Rating Scale) or Sleep-Loss Scale responders/non-responders (defined as ≥2-point improvement in itch interference on sleep).
Results
In ADvocate1 and ADvocate2, significantly greater proportions of itch responders had a clinically meaningful improvement in measures related to QoL (DLQI scores (0/1), ≤5 DLQI total score and ≥4-point DLQI improvement) compared to itch non-responders. In both studies, a significantly greater proportion of Sleep-Loss Scale responders, reported a DLQI score of (0/1), DLQI total score of ≤5 and DLQI improvement of ≥4 points compared to Sleep-Loss Scale non-responders.
Conclusions
Improvement in itch and sleep interference due to itch is associated with improvement in the QoL of patients after treatment with lebrikizumab for moderate-to-severe AD.
ClinicalTrials.gov registration NCT04146363 (ADvocate1) and NCT04178967 (ADvocate2).
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease associated with severe pruritus and inflammatory lesions (Citation1–4). On a global scale, the prevalence of AD is 20% in children (Citation1), and 2% to 7% in adults (Citation5–8). While the onset of AD has been reported in early childhood in 90% of patients (Citation9), a recent meta-analysis suggested that the proportion of adult-onset AD is 26% (Citation10).
A patients’ quality of life (QoL) can be severely impacted due to burdensome symptoms of moderate to-severe AD, particularly increased itch, and sleep interference due to itch (Citation3,Citation11–14). These symptoms can be detrimental to a patients’ QoL and can lead to increased psychological distress and impaired physical and social functioning (Citation3,Citation12,Citation15). Topical corticosteroids are often the first-line anti-inflammatory treatment for patients with AD (Citation16); however, sustained topical therapy use is often inadequate at controlling symptoms, such as itch, and is difficult with larger body surface area (BSA) involvement in patients with moderate-to-severe AD (Citation4,Citation17–20). Recently available advanced systemic treatments, such as biologics and Janus kinase inhibitors, are approved for the treatment of moderate-to-severe AD (Citation21). Primary endpoints of clinical studies related to AD often focus on signs and symptoms related to skin and itch, without considering the impact on the patients’ QoL (Citation22). Therefore, the open question remains whether treatment with lebrikizumab, which improves itch and sleep interference due to itch, leads to additional QoL improvements (Citation22).
Lebrikizumab is an IgG4 monoclonal antibody that binds with high affinity and slow dissociation rate to interleukin (IL)-13 and thereby selectively inhibits IL-13 signaling through the IL-4 receptor alpha (IL-4Rα)/IL-13 receptor alpha 1 (IL-13Rα1) pathway, thus blocking the downstream effects of IL-13 with high potency. Blockade of IL-13 signaling has proven beneficial in IL-13-dominant diseases, such as AD, where it is expressed in peripheral tissues, including the skin, and is implicated in AD pathogenesis, in addition to its role in regulating itch-related receptors and mediators (Citation23–27). Lebrikizumab has been approved in the European Union for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents 12 years and older with a body weight of at least 40 kg who are candidates for systemic therapy.
In two identical randomized, monotherapy Phase 3 trials of patients with moderate-to-severe AD, lebrikizumab improved itch and sleep interference due to itch versus placebo (Citation28). This analysis examines the data from these studies to assess if improvements in itch and sleep interference due to itch are associated with improved QoL in patients.
Materials and methods
Study design
ADvocate1 (NCT04146363) and ADvocate2 (NCT04178967) were identical 52-week randomly assigned, double-blind, parallel-group, placebo-controlled, monotherapy Phase 3 trials (Citation28). Eligible patients included adults (≥18 years old) and adolescents (≥12–<18 years old and weighing ≥40 kg) with moderate-to-severe AD that met an Eczema Area and Severity Index Score of ≥16, an Investigator’s Global Assessment score of ≥3, a body surface area of ≥10%, and had chronic AD for more than one year for which topical treatment was no longer advisable or insufficient at controlling symptoms of AD (Citation29). This analysis focuses on patients from Week 0 to Week 16 and includes data for patients >16 years old.
Eligible patients were randomized 2:1 to either monotherapy lebrikizumab 250 mg (loading dose of 500 mg at baseline and Week 2) or placebo by subcutaneous injection every 2 weeks. ADvocate1 and ADvocate2 studies were approved by the appropriate institutional review boards or ethics committee situated across the 100 study sites in the United States, Canada, Europe, and the Asia/Pacific area. Both studies were conducted in adherence to the Declaration of Helsinki and Good Clinical Practice guidelines.
Itch severity
Itch was measured with Pruritus Numeric Rating Scale (NRS) a patient-reported, single-item, daily, 11-point scale. The Pruritus NRS is used by patients to rate their worst itch intensity over the past 24 h, with 0 indicating ‘No itch’ and 10 indicating ‘Worst itch imaginable’. The minimal clinically important difference (MCID) is 3 points, while a 4-point change is a more conservative assessment of clinical impact (Citation30). Itch responders were defined as having a (weekly mean) ≥4-point reduction in Pruritus NRS from baseline to Week 16.
Sleep interference due to itch
Sleep interference due to itch was assessed using the Sleep-Loss Scale, a patient-reported, single-item, daily scale that measures the extent of Sleep-Loss due to itch over the last night. The Sleep-Loss Scale is rated on a 5-point Likert scale (0 [not at all] to 4 [unable to sleep at all]). The MCID is 1 point, while a 2-point change is a more conservative assessment of clinical impact (Citation31). Sleep-Loss Scale responders were defined as having Sleep-Loss Scale (weekly mean) ≥2 points reduction from baseline at Week 16 in patients with Sleep-Loss Score ≥2 at baseline (Citation30).
Quality of life
The QoL of patients (>16 years of age) was assessed using the Dermatology Life Quality Index (DLQI) questionnaire, a validated, self-administered measure of the impact of AD on QoL (Citation32). The 10-item DLQI questionnaire asks about experiences in the ‘last week’ and covers 6 domains, including symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. Responses are scored from 0 (‘not at all’) to 3 (‘very much’), giving a potential total score ranging from 0 (no impact on QoL) to 30 (maximum impact on QoL), with higher scores indicating a poor QoL. This analysis included the following measures of DLQI in patients with DLQI ≥4 at baseline: DLQI total score of 0 or 1, representing patients for which AD has no impact on QoL; patients with a DLQI total score of ≤5, indicating a small or no effect of AD on QoL (Citation33); and patients who reported ≥4-point DLQI improvement, which is considered the MCID threshold in QoL (Citation34–36).
Statistical analysis
Analyses in ADvocate1 used the intent-to-treat (ITT) population (all randomized patients). In ADvocate2, a total of 18 patients from a single study site were excluded from the ITT population since some or all the study participants did not meet the eligibility criteria of having moderate-to-severe AD. Thus, analyses in ADvocate 2 used the modified intent-to-treat (mITT) population.
The proportion of patients achieving a ≥ 4-point improvement in Pruritus NRS Scores (itch responders) and ≥2-point improvement in Sleep-Loss Scale (Sleep-Loss Scale responders) at Week 16 were compared to itch and Sleep-Loss Scale non-responders, respectively, in both lebrikizumab-treated patients and placebo-treated patient in the ITT (ADvocate 1) and mITT (ADvocate2) populations with baseline Pruritus NRS ≥4 and baseline Sleep-Loss Scale ≥2, respectively.
Patients who received rescue medication or discontinued treatment were identified as non-responders through Week 16. The analysis on binary endpoints were based on logistic regression with treatment, geographic region (US versus EU versus rest of world), age (adolescent patients 12–<18 versus adults ≥18 years), baseline disease severity (IGA 3 versus 4), subgroup, and treatment by subgroup interaction as factors. Missing data were handled by non-responder imputation. Analyses were performed using SAS, Version 9.4 (SAS Institute).
Results
Patient characteristics
A total of 424 and 427 patients were included in the ADvocate1 ITT (lebrikizumab 250 mg: N = 283, placebo: N = 141) and ADvocate2 mITT (lebrikizumab 250 mg: N = 281, placebo: N = 146) populations. Baseline characteristics of the study populations are presented in . Across treatment groups, patients had a mean age of 34–37 years and 48–52% were female. At baseline, 93.7–95.6% of patients reported a Pruritus NRS of ≥4 and 60.1–70.7% of patients reported a Sleep-Loss Scale of ≥2 across treatment groups ().
Table 1. Baseline demographics and disease characteristics.
Treatment effects on itch severity
At Week 16, 53.3% (112/210) and 50.3% (96/191) of lebrikizumab-treated patients reporting a Pruritus NRS score of ≥4 at baseline reported a ≥ 4-point improvement in itch intensity, compared to 20.3% (15/74) and 21.0% (13/62) of placebo-treated patients, in ADvocate1 and ADvocate2, respectively. A significantly higher proportion of lebrikizumab-treated patients reported a ≥ 4-point improvement in itch intensity compared to placebo in both studies (ADvocate1 p < 0.0001 and ADvocate2 p < 0.0001). Data not shown (Citation28).
Impact of itch response on QoL
Significantly greater proportions of itch responders, 42.7% (N = 110) and 34.5% (N = 87), reported DLQI scores (0/1) compared to itch non-responders, 7.6% (N = 224) and 5.0%, (N = 220), or a DLQI total score of ≤5 in itch responders, 74.5% (N = 102) and 67.1% (N = 79), compared to itch non-responders, 21.3% (N = 207) and 19.0% (N = 200), for both ADvocate1 and ADvocate2 studies, respectively (ADvocate1 p = 0.0032; ADvocate2 p < 0.0001; ). Likewise, a significantly greater proportion of itch responders, 93.5% (N = 107) and 96.5% (N = 86), had a clinically meaningful ≥4-point improvement in DLQI compared to itch non-responders, 40.3% (N = 216) and 34.7% (N = 216), for both ADvocate1 and ADvocate2 studies, respectively (ADvocate1: p < 0.0001; ADvocate2: p < 0.0001; ).
Figure 1. Proportion of patients with and without itch improvement (responders and non-responders) achieving each DLQI endpoint after 16 weeks overall or combined lebrikizumab 250 mg and placebo (panels a and b); treated with lebrikizumab 250 mg (panels c and d); and treated with placebo (panels e and f) in ADvocate1 and ADvocate2, respectively. Abbreviations: DLQI: Dermatology Life Quality Index; R: Responder; Non-R: Non-responder; NRI: non-responder imputation; NRS: Numeric Rating Scale. Note. An itch responder (itch improvement) is defined as reporting ≥4-point reduction in Pruritus NRS scores from baseline to Week 16.
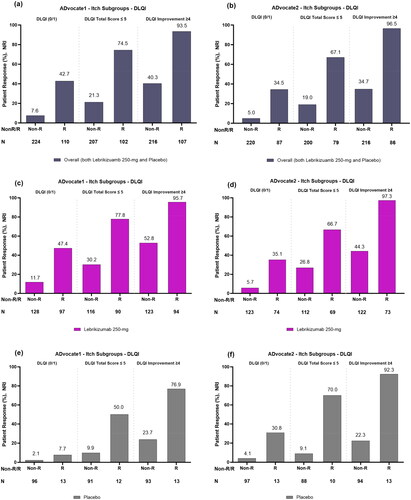
In lebrikizumab-treated patients, a greater proportion of itch responders, 47.4% (N = 97) and 35.1% (N = 74), reported DLQI scores (0/1) compared to itch non-responders, 11.7% (N = 128) and 5.7% (N = 123), for both ADvocate1 and ADvocate2 studies, respectively (). Likewise, a greater proportion of itch responders in this group reported a DLQI total score ≤5, 77.8% (N = 90) and 66.7% (N = 69), compared to itch non-responders, 30.2% (N = 116) and 26.8% (N = 112), for both ADvocate1 and ADvocate2 studies, respectively (). Among treatment groups, a greater proportion of itch responders treated with lebrikizumab, 95.7% (N = 94) and 97.3% (N = 73), had a clinically meaningful ≥4-point improvement in DLQI compared to itch non-responders, 52.8% (N = 123) and 44.3% (N = 122), for both ADvocate1 and ADvocate2 studies, respectively ().
Among placebo-treated patients, a total of 7.7% (n = 1/13) and 30.8% (n = 4/13) itch responders treated with placebo reported DLQI scores (0/1) compared to 2.1% (n = 2/96) and 4.1% (n = 4/97), respectively, of itch non-responders in ADvocate1 and ADvocate2 (). Similarly, 50.0% (n = 6/12) and 70.0% (n = 7/10) of itch responders treated with placebo reported a DLQI total score ≤5 compared to 9.9% (n = 9/91) and 9.1% (n = 8/88) of itch non-responders in ADvocate1 and ADvocate2, respectively (). A total of 76.9% (n = 10/13) and 92.3% (n = 12/13) itch responders treated with placebo reported a clinically meaningful ≥4-point improvement in DLQI compared to 23.7% (n = 22/93) and 22.3% (n = 21/94) of itch non-responders in ADvocate1 and ADvocate2, respectively ().
Treatment effects on sleep interference
After 16 weeks, 46.8% (n = 73/156) and 36.4% (n = 44/121) of lebrikizumab-treated patients reporting a Sleep-Loss Scale of ≥2 at baseline reported a ≥ 2-point improvement in Sleep-Loss Scale, compared to 8.3% (n = 4/48) and 14.0% (n = 6/43) in patients treated with placebo, in ADvocate1 and ADvocate2, respectively. A significantly higher proportion of lebrikizumab-treated patients reported a ≥ 2-point improvement in Sleep-Loss Scale compared to placebo in both studies (ADvocate1 p < 0.0001 and ADvocate2 p = 0.0006). Data not shown (Citation28).
Impact of sleep interference due to itch on QoL
Sleep-Loss responders had a significantly greater proportion of patients who reported a DLQI score of (0/1), 48.6% (N = 70) and 31.7% (N = 41), compared to Sleep-Loss Scale non-responders, 6.1% (N = 179) and 4.1% (N = 169), for ADvocate1 (p = 0.0271) and ADvocate2 (p = 0.0006), respectively. Likewise, a greater proportion of Sleep-Loss Scale responders in this group reported a DLQI total score ≤5, 79.1% (N = 67) and 63.2% (N = 38), compared to Sleep-Loss Scale non-responders, 18.7% (N = 171) and 21.0% (N = 162) for both ADvocate1 and ADvocate2 studies, respectively; ). A significantly greater proportion of Sleep-Loss Scale responders reported DLQI improvement of ≥4 points, 97.1% (N = 69) and 97.6% (N = 41), compared to Sleep-Loss Scale non-responders, 44.9% (N = 176) and 41.9% (N = 167), for both ADvocate1 and ADvocate2 studies, respectively (ADvocate1 p = 0.0003, ADvocate2 p = 0.0005; ).
Figure 2. Proportion of patients with and without sleep improvement (responders and non-responders) achieving each DLQI endpoint after 16 weeks overall or combined lebrikizumab 250 mg and placebo (panels a and b); treated with lebrikizumab 250 mg (panels c and d) and treated with placebo (panels e and f) in ADvocate1 and ADvocate2, respectively. Abbreviations: DLQI: Dermatology Life Quality Index; R: Responder; Non-R: Non-responder; NRI: non-responder imputation. Note: A Sleep-Loss Scale responder (sleep improvement) is defined as reporting a Sleep-Loss Scale ≥2 point reduction from baseline to Week 16. Sleep improvement is improvement of itch interference on sleep.
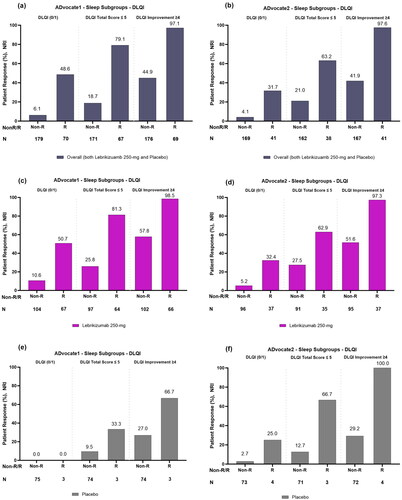
In lebrikizumab-treated patients, a greater proportion of Sleep-Loss Scale responders, 50.7% (N = 67) and 32.4% (N = 37), reported DLQI scores (0/1) compared to Sleep-Loss Scale non-responders, 10.6% (N = 104) and 5.2% (N = 96), for both ADvocate1 and ADvocate2 studies, respectively (). In addition, a greater proportion of Sleep-Loss Scale responders in this group reported DLQI total score ≤5, 81.3% (N = 64) and 62.9% (N = 35) compared to Sleep-Loss Scale non-responders, 25.8% (N = 97) and 27.5% (N = 91), for both ADvocate1 and ADvocate2 studies respectively (). A greater proportion of Sleep-Loss Scale responders treated with lebrikizumab, 98.5% (N = 66) and 97.3% (N = 37) reported a clinically meaningful ≥4-point improvement in DLQI compared to Sleep-Loss Scale non-responders, 57.8% (N = 102) and 51.6% (N = 95), for both studies, respectively ().
Among patients treated with placebo, 0.0% (N = 0/3) and 25.0% (N = 1/4) of Sleep-Loss Scale responders reported DLQI scores (0/1) compared to 0.0% (N = 0/75) and 2.7% (N = 2/73) of Sleep-Loss Scale non-responders in ADvocate1 and ADvocate2 (). Similarly, 33.3% (N = 1/3) and 66.7% (N = 2/3) of Sleep-Loss Scale responders treated with placebo reported a DLQI total score ≤5 compared to 9.5% (N = 7/74) and 12.7% (N = 9/71) of Sleep-Loss Scale non-responders, respectively (). A total of 66.7% (N = 2/3) and 100.0% (N = 4/4) Sleep-Loss Scale responders treated with placebo reported a clinically meaningful ≥4-point improvement in DLQI compared to 27.0%, (N = 20/74) and 29.2% (N = 21/72) of Sleep-Loss Scale non-responders in ADvocate1 and ADvocate2, respectively ().
Discussion
In two identical Phase 3 trials, patients with moderate-to-severe AD, who reported clinically meaningful improvements in itch or sleep interference due to itch, achieved greater improvements in QoL, compared to patients who did not report these itch and sleep improvements. Similar findings have also been reported in the literature for moderate-to severe AD patients (Citation37). Improvement in these key AD symptoms impacted QoL regardless of treatment arm. For Pruritus NRS, in both ADvocate1 and ADvocate2, a higher proportion of responders achieved clinically meaningful improvements in QoL (DLQI 4-point change), small or no impact on QoL (DLQI ≤5) and no impact on QoL (DLQI 0 or 1) endpoints as compared to non-responders. Similarly for the Sleep-Loss Scale, a higher proportion of Sleep-Loss responders achieved all three QoL reported endpoints (DLQI 4-point change, DLQI ≤5, DLQI 0 or 1), as compared to patients that did not report Sleep-Loss improvements. In both ADvocate1 and ADvocate2, a significantly higher percent of lebrikizumab-treated patients reported improvement in itch, sleep interference due to itch, and QoL as compared to placebo (Citation28). Additionally, there were a smaller number of patients in the placebo groups, who experienced itch and sleep improvements. In ADvocate2, itch and sleep responders treated with lebrikizumab or placebo showed similar DLQI responses, however, in ADvocate1, responses were higher for patients treated with lebrikizumab, including little or no impact on QoL (DLQI 0 or 1).
Of note, and consistent across both studies, lebrikizumab itch and sleep non-responders reported greater improvements in QoL compared to placebo non-responders. Therefore, while some patients treated with lebrikizumab may not achieve a clinically significant impact in Pruritus NRS or Sleep-Loss Score as of week 16, there remains a beneficial impact on QoL in these patients. This may point toward additional lebrikizumab treatment effects, such as reduction of skin inflammation and skin pain that also impact QoL (Citation38). Furthermore, the relief that lebrikizumab provides from the burdensome symptoms of itch and its resulting interference on sleep, is important to consider as these are often the main symptoms that reduce patient QoL (Citation28,Citation39).
Limitations
DLQI data reported in this study only include patients with moderate-to-severe AD that were >16 years old. Further analysis could provide insights into how improvements in itch and sleep interference due to itch might improve QoL in younger patients, after treatment with lebrikizumab. In addition, the analyses presented in this study are post hoc and thus were not adjusted for multiplicity.
Conclusion
This study demonstrates that improvements in itch and sleep interference due to itch have an impact on DLQI measures in patients after lebrikizumab treatment for moderate-to-severe AD. Moreover, lebrikizumab seems to exert additional benefit on QoL beyond reducing itch. This study highlights the key importance of assessing and reducing these symptoms, with the overall goal to enhance the lives of patients with AD.
Ethical approval
Informed consent was obtained from all patients before study procedures were initiated. For patients considered to be minors, the written consent of the parent or legal guardian, as well as the assent of the minor, was obtained.
Acknowledgments
We gratefully acknowledge the contributions of the patients who participated in this study. Sarah Ryan of Eli Lilly and Company provided medical writing and editorial assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank, or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Additional information
Funding
References
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):1–9. doi:10.1016/S0140-6736(20)31286-1.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–151. doi:10.1016/j.anai.2019.04.020.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Investig Dermatol. 2017;137(1):26–30.
- Bieber T, Straeter B. Off-label prescriptions for atopic dermatitis in Europe. Allergy. 2015;70:6–11.
- Harrop J, Chinn S, Verlato G, et al. Eczema, atopy and allergen exposure in adults: a population-based study. Clin Exp Allergy. 2007;37(4):526–535. doi:10.1111/j.1365-2222.2007.02679.x.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173(6):1411–1419. doi:10.1111/bjd.14151.
- Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):595–605. doi:10.1016/j.clindermatol.2018.05.007.
- Saeki H, Tsunemi Y, Fujita H, et al. Prevalence of atopic dermatitis determined by clinical examination in Japanese adults. J Dermatol. 2006;33(11):817–819. doi:10.1111/j.1346-8138.2006.00187.x.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi:10.1038/s41572-018-0001-z.
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526–1532 e7. doi:10.1016/j.jaad.2018.05.1241.
- Laughter MA-O, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–309.
- Pompili M, Bonanni L, Gualtieri F, et al. Suicidal risks with psoriasis and atopic dermatitis: systematic review and meta-analysis. J Psychosom Res. 2021;141:110347.
- Slattery M, Fau- Essex MJ, Essex M, et al. Depression, anxiety, and dermatologic quality of life in adolescents with atopic dermatitis. J Allergy Clin Immunol. 2011;128(3):668–671.
- Narla S, Silverberg JI. The role of environmental exposures in atopic dermatitis. Curr Allergy Asthma Rep. 2020;20(12):74. doi:10.1007/s11882-020-00971-z.
- Andersen L, Nyeland ME, Nyberg F. Increasing severity of atopic dermatitis is associated with a negative impact on work productivity among adults with atopic dermatitis in France, Germany, the U.K. and the U.S.A. Br J Dermatol. 2020;182(4):1007–1016.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema - part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904–1926. doi:10.1111/jdv.18429.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682. doi:10.1111/jdv.14891.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878. doi:10.1111/jdv.14888.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744. doi:10.1111/jdv.16892.
- Sidbury R, Kodama S. Atopic dermatitis guidelines: diagnosis, systemic therapy, and adjunctive care. Clin Dermatol. 2018;36(5):648–652. doi:10.1016/j.clindermatol.2018.05.008.
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21(1):21–40. doi:10.1038/s41573-021-00266-6.
- Patrizi A, Costanzo A, Patruno C, et al. Unmet needs in atopic dermatitis management: an expert consensus. J Dermatolog Treat. 2022;33(5):2459–2465. 4 doi:10.1080/09546634.2021.1967267.
- Ratnarajah KA-O, Le M, Muntyanu AA-OX, et al. Inhibition of IL-13: a new pathway for atopic dermatitis. J Cutan Med Surg. 2020;25:315–328.
- Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75(1):54–62. doi:10.1111/all.13954.
- Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863–871 e11. doi:10.1016/j.jaad.2018.01.017.
- Ungar B, Garcet S, Gonzalez J, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2017;137(3):603–613. doi:10.1016/j.jid.2016.09.037.
- Xiao S, Lu Z, Steinhoff M, et al. Innate immune regulates cutaneous sensory IL-13 receptor alpha 2 to promote atopic dermatitis. Brain Behav Immun. 2021;98:28–39. doi:10.1016/j.bbi.2021.08.211.
- Silverberg JI, Guttman-Yassky E, Thaçi D, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388(12):1080–1091. doi:10.1056/NEJMoa2206714.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351. doi:10.1016/j.jaad.2013.10.010.
- Revolutionizing Atopic Dermatitis, 11–13 December 2021. Br J Dermatol. 2022;186(4):e135–e173.
- Yosipovitch G. Revolutionizing atopic dermatitis, 11–13 December 2021. Br J Dermatol. 2022;186(4):e147.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x.
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664. doi:10.1111/j.0022-202X.2005.23621.x.
- Silverberg JI, Margolis DJ, Boguniewicz M, et al. Validation of five patient-reported outcomes for atopic dermatitis severity in adults. Br J Dermatol. 2020;182(1):104–111. doi:10.1111/bjd.18657.
- Khilji FA, Gonzalez M, Finlay A. Clinical meaning of change in dermatology life quality index scores. Br J Dermatol. 2002;147:50.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi:10.1159/000365390.
- Lio PA, Simpson EL, Han G, et al. Improvement in sleep and itch and enhanced quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a phase 3 trial of baricitinib therapy. J Dermatolog Treat. 2022;33(4):2057–2062. doi:10.1080/09546634.2021.1914308.
- Koszoru K, Borza J, Gulacsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104(3):174–177.
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-Affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156(4):411–420. doi:10.1001/jamadermatol.2020.0079.

