Abstract
Background
Data on the characteristics and treatment outcomes of super-responders and non-super-responders in psoriasis under adalimumab treatment are limited.
Methods
A retrospective analysis from psoriatic patients treated with adalimumab was compared to characterize super-responders vs non-super-responders’ groups, identify factors associated with super response, and assess treatment outcomes after switching.
Results
15 out of 70 (21.4%) patients were categorized as super-responder. The proportion of patients achieving a PASI 100 response was significantly higher in super-responders than non-super-responders at weeks 12, 24, and 52. Female sex and Charlson Co-morbidity Index were significantly associated with super-responders. A high level of high-density lipoprotein was independently associated with PASI 90 response at weeks 24 and 52. Additionally, nearly 35%–43% of non-super-responders switching to interleukin-17A (IL-17A) inhibitors may achieve a PASI 100 response at week 12. In contrast, all super-responders switching to IL-17A inhibitors achieved a PASI 100 response at week 4.
Conclusions
Super-responders treated with adalimumab have a higher rate of being female and fewer comorbidities. And super-responders have better PASI responses than non-super-responders, whether the patients were treated with adalimumab or switched to IL-17A inhibitors.
Introduction
Psoriasis is a chronic, systemic, immune-mediated disease affecting an estimated 125 million people worldwide (Citation1). Due to its nature of a painful, disfiguring and disabling disease, most patients with psoriasis suffered an detrimental impacts on their quality of life and psychological health (Citation2,Citation3).
Biologics have drastically improved the short-term and long-term outcomes of psoriasis (Citation4). Recently, the standard in therapeutic efficacy in psoriasis has shifted more focus on achieving complete skin clearance, which reflects no psoriasis symptoms and no impairment in quality of life (Citation5). Patients that present a fast skin clearance with treatment are called super-responders (SR) (Citation6), but not all patients respond in the same manner. Non-super-responders (NSR) might not respond to the given treatment (primary failure) or, more commonly, lose the initial response (secondary failure) (Citation7). Although there are a number of studies reporting on SR in the biologic treatment of psoriasis, most of the work have focused on interleukin (IL)-17A and IL-23 inhibitors (Citation7–10). Clinical data identifying characteristics of SR, compared with NSR treated with adalimumab, is lacking.
Adalimumab, one of the oldest of biologics for the treatment of psoriasis, is a full human, anti-tumor necrosis factor (TNF)-α monoclonal antibody (Citation11). Although a randomized, head-to-head study provided evidence that IL-17 inhibitors offer higher responses than adalimumab across musculoskeletal and skin endpoints (Citation12), an updated network meta-analysis found that adalimumab was comparable with respect to high short-term efficacy and tolerability among biologics (Citation13). Additionally, patients with an inadequate response to initial treatment might benefit from switching to other blocking agents (Citation14,Citation15). Few studies have investigated the treatment trajectory or outcomes of adalimumab-treated patients after discontinuation or switching to other biologics.
Identifying the predictors or outcomes for SR can play a pivotal role in selecting the most appropriate treatment for psoriasis patients. In this study, we determined the baseline characteristics, treatment responses, safety, drug survival and treatment trajectory of adalimumab-treated patients who were SR vs those who were NSR in a real clinical practice setting.
Materials and methods
Study design
This is a single-center, longitudinal retrospective analysis of real-world pilot series including patients with psoriasis treated with adalimumab. This study retrospectively analyzed information including patients treated with adalimumab for psoriasis between September 2019 and June 2022 at the Psoriasis Specialty Clinic of Xiangya Hospital. The study followed the Declaration of Helsinki and was approved by the Institutional Research Ethics Committee of Xiangya Hospital (202308636). Patients gave informed consent before inclusion in the study.
Patients
Patients treated with adalimumab were included if they met one of the following inclusion criteria: (1) Psoriasis area and severity index (PASI) score ≥ 3 at baseline; (2) dermatology life quality index (DLQI) score > 5 at baseline; (3) patients with psoriasis arthritis (Citation16). In all cases, adalimumab was administrated subcutaneously 80 mg at week 0, and then 40 mg every two weeks during the treatment period (Citation17). Exclusion criteria were a biologic indication other than psoriasis, psoriasis arthritis patients without skin involvement and lost follow-up before week 52.
Definition of SR and NSR
There is no consensus on the definition of SR in psoriasis (Citation6), therefore, based on the relevant literature and our real-life experience, we define SR as patients who achieved PASI 100 response at week 12 and achieved the response at week 24 or 32 at least once based on a PASI score <1. NSR is defined as all patients included in the study except for SR.
Outcome measures
The effectiveness endpoints were the improvement of 75%, 90%, and 100% in PASI compared with baseline score (PASI75, PASI90, and PASI100, respectively) (Citation18).The definition of drug survival was similar with previous studies, with discontinuation of treatment for more than 90 days (Citation19).
Statistical analysis
SR versus NSR PASI response data of patients who received adalimumab were presented from week 4 through week 52. The PASI 75, 90, and 100 responses were examined between SR and NSR groups to compare the speed and stability of response to adalimumab. Effectiveness data were summarized through 52 weeks using as observed method. In the regression analysis, patients who discontinued adalimumab treatment due to ineffectiveness and adverse events (AEs) were considered as being a NSR status.
Descriptive summary of demographics and baseline disease characteristics were compared between SR and NSR. Asian patients are diagnosed of metabolic syndrome (Mets) if meeting 3 or more of the following criteria: central obesity (waist circumference ≥ 90 cm for men and ≥ 80 cm for women); low high density lipoprotein (HDL, <1.03 mmol/L for men and <1.29 mmol/L for women); hypertriglyceridemia (≥1.7 mmol/l); high fasting glucose (≥5.6 mmol/L); and high blood pressure (≥130/85 mmHg) (Citation20). The Charlson Co-morbidity Index (CCI) was used as an index of comorbidities (Citation21). The chi-square and Fisher exact tests were used to compare categorical variables, and for continuous variables the Student t-test and Mann–Whitney U-test were used for comparison.
To investigate which variables were independent predictive of SR, multifactorial logistic regression analysis was performed on all variables with p < .2 in the univariate analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported. Drug survival was determined using Kaplan–Meier plot analysis. A log-rank test was used to compare the adalimumab survival of patients in the SR and NSR. All analyses were performed using SPSS 26.0 and GraphPad Prism version 8.0.0.
Results
In total, 70 patients were included: 15 adalimumab-treated patients identified as SR and 55 adalimumab-treated patients identified as NSR.
Demographics and disease characteristics, laboratory data, safety evaluation of SR vs NSR
The demographic, disease characteristics, and laboratory data at baseline of psoriasis cohort, classified according to their response to adalimumab, are given in . SR displayed a lower proportion of patients with CCI ≥1 (20.0% vs 61.8%, p < .01) than NSR. Specifically, significant differences were observed between SR and NSR groups in terms of previous biological treatment (0 vs 29.1%, p < .05). There were no significant differences in age at diagnosis of psoriasis, disease durations, cigarette use between the two groups. All other variables, such as weight, body mass index (BMI), initial PASI score, laboratory examinations, were not found to be significantly different between SR and NSR. NSR were characterized by higher laboratory examinations values (except for HDL values) (). Most adverse reactions (AEs) were mild to moderate in severity and comparable in SR and NSR groups (13.3% vs 14.5%). Interestingly, none of the AEs led to the discontinuation of treatment in SR group. But in NSR group, there were two patients that discontinued adalimumab due to conjunctivitis at week 84 and cough at week 124, respectively ().
Table 1. Baseline demographic and clinical characteristics.
Table 2. Safety outcomes between SR and NSR.
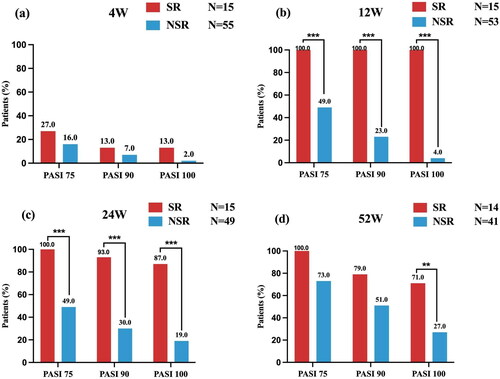
Comparison of PASI response between SR and NSR patients
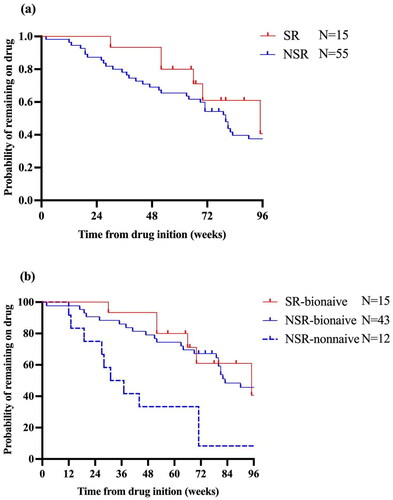
The fast and continuous effectiveness of adalimumab treatment in SRs were observed, as PASI 100 was achieved by 13% of the SR at week 4, 100% at week 12, 87% at week 24, and 71% at week 52. PASI 90 response was achieved by 13%, 100%, 93%, and 79% of SR at the same time points (). Between weeks 4, 12, and 24, the proportions of patients with PASI 75, 90, and 100 responses in the NSR were lower than those in the SR group. After week 24, the difference in the proportion of patients with PASI response favoring SR over NSR decreased over time (). However, the PASI 100 response rates between two groups were still significantly different until week 52.
Figure 1. Comparison of PASI response at weeks 4, 12, 24, and 52 between SR and NSR. bIn the NSR group, one patient discontinued the adalimumab treatment and one patient switched to secukinumab. cIn the NSR group, another four patients switched to secukinumab (n = 3) and stekinumab (n = 1), respectively. Among 15 super- responders, 2 (13.3%) patients had a mild degree of psoriasis (PASI = 0.5; PASI =0.6) at week 24 and reached a PASI 100 response again at week 32. dIn the SR group, one patient discontinued the adalimumab treatment, while in the NSR group, eight additional patients, discontinued (n = 4) and switched to IL-17A inhibitors (seckinumab, n = 2; ixekizumab,n = 2), respectively. SR: super responder; NSR: non-super responder; PASI: psoriasis area and severity index. **p-value < .01; ***p-value < .001.
Predictors of super-response statue to adalimumab
Categorical variables revealed that male, CCI ≥1, with Mets, low HDL, and prior biologic experience was associated with reduced odds of achieving PASI 90 or 100 response at week 24 or 52 (). The univariate analyses assessing the association between variables and the attainment of PASI 90 or 100 response identified five variables with p < .2 that were taken forward to the multivariable analysis (). Mets was excluded due to high correlation with HDL. After 24 weeks, male patients (OR, 0.21; CI 0.05–0.95, p < .05) or patients with CCI ≥1 (OR, 0.22; CI 0.06–0.76, p < .05) were independently associated with lower PASI 100 response. Similarly, low HDL was independently associated with worse response in terms of PASI 90 at week 24 (OR, 0.24; CI 0.07–0.79, p < .05) and 52 (OR, 0.13; CI 0.04–0.44, p < .01). Additional negative predictor of PASI 90 was previous biological exposure (OR, 0.08; CI 0.01–0.81, p < .05) at week 52.
Figure 2. Forest plot of variables associated with the likelihood of reaching PASI 90 and PASI 100 at week 24 and week 52. HDL: high-density lipoprotein; CCI: Charlson Co-morbidity Index; PASI: psoriasis area and severity index; Y/N: yes versus no; M/F: male versus female.
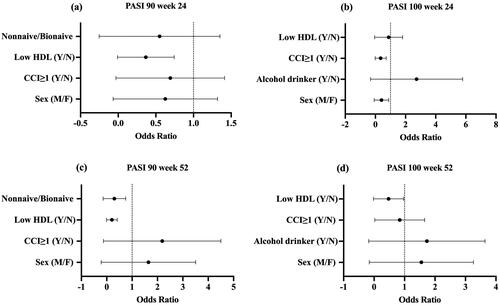
Table 3. Univariate logistic regression analysis: potential predictors for SR.
Drug survival in SR and NSR patients
Broadly, SR had higher rate of drug survival than NSR at all timepoints, but without statistical significance (p = .23, ). We stratified drug survival in the NSR cohorts by bio-naïve status. There was significantly lower drug survival in the non-bio-naïve cohort compared with bio-naïve cohort (SR-bio-naïve vs NSR-nonnaïve, p < .01; NSR-bio-naïve vs NSR-nonnaïve, p < .01, ), while for bio-naïve cohorts there was no significant difference between SR and NSR groups (p = .55). Analysis of reasons for discontinuation or switching showed that the primary reason was loss of effectiveness.
Treatment trajectory for NSR patients, and early effectiveness of switching to other therapeutics in SR and NSR patients
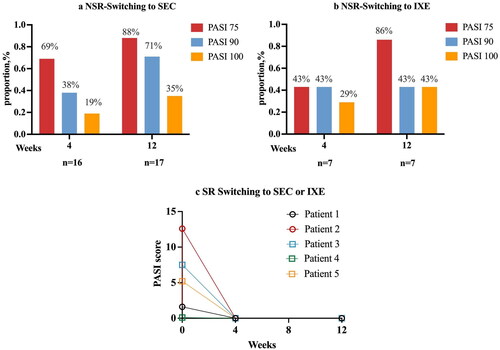
The adalimumab treatment was terminated due to treatment failure at week 120 in 80% NSR patients. After week 96, NSR patients who switched to other biologicals constituted the largest part of NSR group (44% NSR switching to other biologicals at week 96, and 60% at week 120, Supplemental Figure 1). The frequency of discontinuation or drug switching in SR were numerically lower than in NSR patients, but half of SR data were censored.
After switching to seckinumab, 19% of patients attaining PASI 100 at week 4. At week 12, 15 patients (88%) achieved PASI 75, 12 (71%) achieved PASI 90 and 6 (35%) achieved PASI 100. Of the eight patients who switched to ixekizumab, two (29%) patients achieved PASI 100 at week 4, and three patients (43%) achieved PASI 100 at week 12. Interestingly, 100% of SR who switched to IL-17 inhibitors achieved PASI 100 at weeks 4 and 12 ().
Figure 4. Early effectiveness of NSR and SR after IL-17A inhibitors switching therapy. aPatients who switched to SEC were administrated subcutaneously (150 mg for patients < 60 kg and 300 mg for patients > 60 kg) at weeks 0, 1, 2, 3, and 4, and then every four weeks. bThe dosing regimen for patients who switching to IXE is 160mg at week 0, then 80 mg every 2 weeks for 12 weeks. cIn ©, patients 1 and 2 switched to IXE, patients 3–5 switched to SEC. SR: super responder; NSR: non-super responder; IXE: ixekizumab; SEC: secukinumab.
Discussion
We assessed whether there were differences in clinical characteristics, treatment outcomes and drug survival rate between SR and NSR treated with adalimumab. Using a logistic regression to analyze the characteristics considered to be the most clinically relevant, we showed that female, higher HDL and fewer comorbidities were associated with patients achieving a super-response status. In addition, we found that patients without previous biologic exposure were more likely to achieve almost clearance. Analyses also determined that SR had a higher likelihood of maintaining PASI 75/90/100 responses at week 52 when compared with NSR. In line with this, SR were more likely to achieve clearance than NSR after switching to IL-17A inhibitors. Although no significant differences in drug survival rate were observed between adalimumab-treated SR and NSR, our research revealed that drug survival was statistically significantly better in patients without biologic exposure (p < .01).
Here, we found two independent variables associated with SR, female sex, and comorbidities, which were consistent with previous evidence. Two prior studies found female sex to be associated with increased effectiveness (Citation22,Citation23), which is further supported by our data. Sex differences in the effectiveness are attributed to hormonal factors (Citation24). In particular, production of TNF-α is modulated by sex hormones and is increased in males (Citation25). In our logistics regression model, the absence of comorbidities was associated with increase in SR. A nationwide study also reported that a smaller proportion of SR had any comorbidities from the CCI compared with NSR (Citation7).
In our experience, the most pronounced predictor was observed for HDL, among all patients, low HDL was associated with 87% reduced odds of achieving PASI 90 response (OR, 0.13, 95% CI, 0.04–0.44) at week 52. Prior data assessing the association between hyperlipidemia and treatment response yielded conflicting results. A prospective analysis in Corrona Psoriasis Registry found no correlation between hyperlipidemia and treatment response during a six-month follow-up period, but the associations of obesity and diabetes with treatment outcome were strong (Citation26). In contrast, a recent study using the CorEvitas Psoriasis Registry found that a history of hyperlipidemia was associated with over three-fold increase in multiple biologic failure (Citation27). The observed results may be explained by the systemic inflammatory milieu of psoriasis possibly contributing to inferior effectiveness. Further research still needs to be conducted to confirm association between HDL level and biological effectiveness.
Additional variables, such as prior use of biological therapy, was associated with decreased odds of achieving PASI 90 response and exhibited lower drug survival rate. The negative effect of prior biologic exposure in effectiveness and adherence has also been highlighted in other studies (Citation9). Nevertheless, SR showed better persistence and lower discontinuation over 52 weeks compared to NSR. Recently, the association of complete skin clearance and drug survival of biologics have received substantial attention. The finding showed that complete skin clearance is associated with increased drug survival and a reduced need for drug switching (Citation28). Similar findings have been observed in our results. We also performed an extended follow-up to 12 weeks after the patients switching to IL-17A inhibitors. For those who were treated with adalimumab did not achieve satisfied response, ixekizumab and secukinumab were shown to be two valid treatment options. Interestingly, proportionally more SR patients achieved PASI 100 at weeks 4 and 12 compared with NSR after switching to IL-17A inhibitors. This was further proof that IL-17A inhibitors were effective for patients with prior exposure to adalimumab, especially for SR of adalimumab. Above all, these findings further represented that SR patients not only achieved fast and great response to adalimumab but also maintained long and stable outcome.
This is a retrospective cohort study using real-world data to undertake a direct-comparison study between SR and NSR with psoriasis under adalimumab treatment. These results broaden the insights of a prior analysis wherein baseline characteristics, treatment responses, and persistence among SR and NSR were compared. The study is mainly limited by its nature of retrospective, real-world and the small sample size. Some data were missing, including that of treatment responses at weeks 12, 24, and 52, and treatment trajectory over 52 weeks of SR and NSR.
In summary, those who were SR treated with adalimumab were more likely to be female, had higher HDL and fewer comorbidities. SR patients maintained PASI 100 response over 52 weeks and have numerically longer drug survival rate. Nevertheless, in case of ineffectiveness to adalimumab, SR patients switching to IL-17A inhibitors would achieve better response compared with NSR patients.
Ethical approval
This work was reviewed and approved by Xiangya Hospital; approval #: 202308636
Consent form
The written informed consent to participate was obtained from participants.
Supplemental Material
Download MS Word (197.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1–8. doi: 10.1001/jama.2020.4006.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984–991. doi: 10.1038/jid.2014.530.
- Gisondi P, Puig L, Richard MA, et al. Quality of life and stigmatization in people with skin diseases in Europe: a large survey from the ‘burden of skin diseases’ EADV project. J Eur Acad Dermatol Venereol. 2023;37 Suppl 7:6–14. doi: 10.1111/jdv.18917.
- Ghoreschi K, Balato A, Enerbäck C, et al. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi: 10.1016/S0140-6736(21)00184-7.
- Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77–82 e7. doi: 10.1016/j.jaad.2016.03.026.
- Thomas SE, van den Reek J, Seyger MMB, et al. How to define a ‘super-responder’ to biologics in psoriasis studies. Br J Dermatol. 2023;189(5):621–622. doi: 10.1093/bjd/ljad280.
- Loft N, Egeberg A, Rasmussen MK, et al. Prevalence and characterization of treatment-refractory psoriasis and super-responders to biologic treatment: a nationwide study. J Eur Acad Dermatol Venereol. 2022;36(8):1284–1291. doi: 10.1111/jdv.18126.
- Reich K, Gordon KB, Strober B, et al. Super-response to guselkumab treatment in patients with moderate-to-severe psoriasis: age, body weight, baseline psoriasis area and severity index, and baseline investigator’s global assessment scores predict complete skin clearance. J Eur Acad Dermatol Venereol. 2022;36(12):2393–2400. doi: 10.1111/jdv.18474.
- Gargiulo L, Ibba L, Malagoli P, et al. A risankizumab super responder profile identified by long-term real-life observation-IL PSO (ITALIAN LANDSCAPE PSORIASIS). J Eur Acad Dermatol Venereol. 2023;38(1):e113–e116. doi: 10.1111/jdv.
- Ruiz-Villaverde R, Rodriguez-Fernandez-Freire L, Armario-Hita JC, et al. Super responders to guselkumab treatment in moderate-to-severe psoriasis: a real clinical practice pilot series. Int J Dermatol. 2022;61(8):1029–1033. doi: 10.1111/ijd.15784.
- Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606. doi: 10.1016/j.jaad.2006.05.027.
- Gottlieb AB, Merola JF, Reich K, et al. Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study. Br J Dermatol. 2021;185(6):1124–1134. doi: 10.1111/bjd.20413.
- Mahil SK, Ezejimofor MC, Exton LS, et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol. 2020;183(4):638–649. doi: 10.1111/bjd.19325.
- Gasslitter I, Kirsten N, Augustin M, et al. Successful intra-class switching among IL-17 antagonists: a multicentre, multinational, retrospective study. Arch Dermatol Res. 2019;311(5):421–424. doi: 10.1007/s00403-019-01907-y.
- Conti A, Peccerillo F, Amerio P, et al. Efficacy and safety of switching to ixekizumab in secukinumab nonresponder patients with psoriasis: results from a multicentre experience. Br J Dermatol. 2019;180(6):1547–1548. doi: 10.1111/bjd.17580.
- Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117–122. doi: 10.1016/j.jaad.2019.08.026.
- Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89–95. doi: 10.1111/jdv.13746.
- Gisondi P, Fargnoli MC, Amerio P, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol. 2022;157(Suppl. 1 to No. 1):1–78. doi: 10.23736/S2784-8671.21.07132-2.
- Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–2640. doi: 10.1038/jid.2015.208.
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/s0140-6736(05)67402-8.
- Geale K, Henriksson M, Jokinen J, et al. Association of skin psoriasis and somatic comorbidity with the development of psychiatric illness in a nationwide Swedish study. JAMA Dermatol. 2020;156(7):795–804. doi: 10.1001/jamadermatol.2020.1398.
- Do anti-psoriatic drugs work better in women than men? Br J Dermatol. 2021;185(6):e200–e13. doi: 10.1111/bjd.20787.
- Menendez Sanchez M, Muniz de Lucas A, Perez Fernandez E, et al. Super-responders in psoriasis under interleukin 23 inhibitor treatments, experience in two centres. J Eur Acad Dermatol Venereol. 2023;37(11):e1321–e1322. doi: 10.1111/jdv.19310.
- Wilkinson NM, Chen HC, Lechner MG, et al. Sex differences in immunity. Annu Rev Immunol. 2022;40(1):75–94. doi: 10.1146/annurev-immunol-101320-125133.
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90.
- Enos CW, Ramos VL, McLean RR, et al. Comorbid obesity and history of diabetes are independently associated with poorer treatment response to biologics at 6 months: a prospective analysis in Corrona Psoriasis Registry. J Am Acad Dermatol. 2022;86(1):68–76. doi: 10.1016/j.jaad.2021.06.883.
- Jin JQ, Cronin A, Roberts-Toler C, et al. Sociodemographic and clinical characteristics associated with multiple biologic failure in psoriasis: a 2015-2022 prospective cohort analysis of the CorEvitas Psoriasis Registry. J Am Acad Dermatol. 2023;89(5):974–983. doi: 10.1016/j.jaad.2023.06.058.
- Song WJ, Yoon HS. Association between complete skin clearance (psoriasis area and severity index 100) and drug survival of biologics in patients with chronic plaque psoriasis: a single-center retrospective study. J Am Acad Dermatol. 2023;89(4):848–851. doi: 10.1016/j.jaad.2023.06.022.