Abstract
The purpose of the article: Generalized pustular psoriasis (GPP) is a rare auto-inflammatory disease. Patients with GPP may develop life-threatening complications, including sepsis, acute renal failure, neutrophilic cholangitis, high-output congestive heart failure, acute respiratory distress syndrome and death. The therapy of GPP is very limited and the course of the disease is unpredictable.Materials and methods: We report a 60-year-old woman presenting with widespread and confluent erythematous-desquamative plaques with numerous small pustules covering almost 70% of the body surface area. Over the past years patient had undergone different types of conservative treatment regimens including topical therapy, acitretin, cyclosporin, methotrexate and long-term treatment with systemic corticosteroids. Considering the patient’s overall clinical condition, we proceed to initiate the biologic therapy with guselkumab.Results: Guselkumab (anti-IL-23) in the standard dose of 100 mg was administered subcutaneously at weeks 0, 4 and followed by a maintenance dose every 8 weeks. The remission of GPP was observed already after 12 weeks of treatment. The maintenance treatment in the period of 18 months shows stable clinical response.Conclusions: Our results support the evidence that guselkumab could provide an effective therapeutic approach in the treatment of GPP.
Introduction
Generalized pustular psoriasis (GPP) is a rare and potentially life-threatening auto-inflammatory disease characterized by recurrent episodes of widespread painful erythema with sterile pustules (Citation1,Citation2). It is clinically heterogenous disease with a highly variable clinical course. There are several GPP subtypes, which are subdivided into GPP and localized palmoplantar pustular psoriasis (Citation3). GPP is an unpredictable disease with a highly variable clinical course where the extent and severity of symptoms vary between patients, even between each flare. Patients with GPP may develop life-threatening complications, including sepsis, acute renal failure, neutrophilic cholangitis, high-output congestive heart failure, acute respiratory distress syndrome and death (Citation4). The therapy of GPP is very limited. Currently, there is no fully available GPP-specific therapy. Disease is usually refractory to many anti-psoriatic drugs. Retinoids, cyclosporine, and methotrexate are commonly used non-biologic therapies for GPP with variable efficacy (Citation5).
Materials and methods
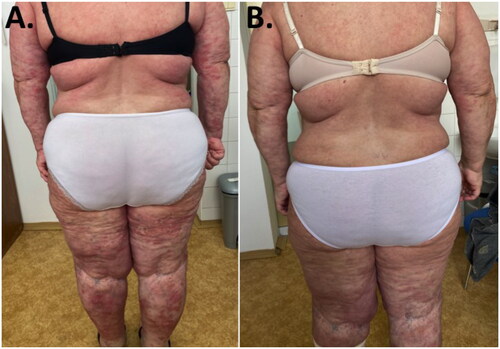
We report a 60-year-old woman presenting with widespread and confluent erythematous-desquamative plaques with numerous small pustules covering almost 70% of the body surface area (PGA 3, DLQI 26). The patient was suffering from GPP for 15 years. She was treated for heart failure (NYHA III) and moderate psoriatic arthritis (DAS28-CRP 3.39) with sacroiliitis (stage III-IV) and bilateral plantar fasciitis. In addition, patient complained of recurrent fever, skin and joints pain. Based on medical history patient had undergone different types of conservative treatment regimens including topical therapy (moderate/high potency corticosteroids, tacrolimus), acitretin (50 mg daily for 3 years), cyclosporin (4 mg/kg body weight for 12 months), methotrexate (20 mg per week for 5 years) and long-term treatment with systemic corticosteroids (0,25 − 1 mg/kg body weight) (). Most of them provided only limited clinical benefit. Only use of the corticosteroids reportedly provided relatively satisfactory clinical response. The systemic corticosteroids were used either as a monotherapy or as a concomitant medication with anti-psoriatic drugs and their withdrawal always led to deterioration and worsening of the disease. Due to prolonged corticosteroids overuse, she developed adverse events, such as hyperglycemia, increase of body weight, uncontrolled hypertension and muscle weakness.
Results
Considering the patient’s overall clinical condition, we initiated biologic therapy. Since the presence of heart failure contraindicated the use of TNF-α inhibitors therefore we decided to use interleukin inhibitor. In Slovakia, guselkumab (anti-IL-23) is the only interleukin IL-23 inhibitor approved for the treatment of moderate to severe plaque psoriasis in biologically naive patients. Guselkumab in the standard dose of 100 mg was administered subcutaneously at weeks 0, 4 followed by a maintenance dose every 8 weeks. Clinical evaluation was performed 3 months after the first dose (). Significant resolution of pathological skin changes was observed, with initial PGA 3 decreased to PGA 0. The quality of life of the patient improved following the treatment from DLQI 26 to DLQI 4. Also, the improvement of psoriatic arthritis was observed. DAS28-CRP score decreased from 3.39 to 2.17, which means achieving a low disease activity. Based on improved clinical condition of the patient corticosteroids were tempered down and discontinued in 4 weeks after guselkumab therapy initiation. Neither rebound phenomenon nor new GPP flares were observed following corticosteroids detraction.
Discussion
The pathogenic mechanism of GPP is not completely understood and therapeutic intervention in GPP still represents a significant challenge. Despite of partial overlap between the pathological pathways of GPP and plaque psoriasis recent advances in understanding of molecular mechanisms of autoinflammation and autoimmunity indicates that GPP is a distinct entity from plaque psoriasis, with different cytokine pathways predominant in the manifestation of each disease. While TNF-α, IL-23/IL-17, IL-22 axis is predominantly driving the plaque psoriasis, an interleukin-36 (IL-36) signaling cascade appears to be a key driver in the pathogenesis of GPP (Citation5,Citation6). The central role of IL-36 pathway in the pathogenesis of GPP is supported by the presence of IL36RN mutations in patients with GPP. Mutations of IL36RN leads to the synthesis of abnormal IL-36 receptor antagonist (IL36Ra), which is not able to inhibit the proinflammatory activities of IL-36 thus allowing uninhibited IL-36 signaling leading to the clinical manifestations of GPP (Citation5,Citation6). The higher prevalence of IL36RN mutation was described in Japanese patients with GPP compared to countries in the world (Citation7).
The investigation of biologic agents targeting the IL-36 pathway have demonstrated promising efficacy in patients with GPP and thus potentially a new era of targeted therapy for GPP. Spesolimab is the first anti-IL-36 receptor monoclonal antibody approved for the treatment of GPP (Citation8).
Since all signaling cascades closely interact, the proinflammatory functions of IL-36 cytokines can be further potentiated by a positive feedback loop with the IL-17/IL-23 axis perpetuating inflammatory response (Citation6,Citation9). Therefore the biologics, known for being effective in the treatment of plaque psoriasis, could be also used in GPP. Recently, The Japanese Society for Psoriasis Research has performed epidemiological analysis of patients with GPP from 2017 - 2020. In this analysis they evaluated therapeutic trends in all types of pustular psoriasis in Japan on a total of 291 patients with pustular psoriasis. The most commonly occurring type of pustular psoriasis was von Zumbusch type (59.8%). According to this survey, the majority of the patients with all types of pustular psoriasis (58,4%) were treated with oral medication mostly by etretinate, corticosteroids and cyclosporine. The biologics were used only in 44% of cases. The most commonly used biologics were IL-17 inhibitors, tumor necrosis factor inhibitors and IL-23 inhibitors (Citation10).
Since spesolimab is not yet currently fully available in all countries, there is still an unmet clinical need for alternative therapeutic approach. The treatment with the guselkumab, was already suggested as an effective therapy in patients with GPP. According to the Chinese single center observational study of 50 patients with GPP, guselkumab was the most potent drug, although not primary approved for the therapy of this disease. The other studied biologics (adalimumab, secukinumab) resulted in lower rate of clinical improvement. After 12 weeks of treatment, guselkumab provided the most effective response rate compared to adalimumab and secukinumab. The authors of the study established that the suppression of tissue resident memory T cells (TRM) is crucial in the treatment of GPP. The number of TRM cells was higher in pustular psoriatic skin lesions than in normal control lesions. The number of CD8+ TRM cells in dermis and epidermis decreased after guselkumab therapy (Citation11).
The successful treatment with guselkumab was also described in a multicenter, open-label study (a phase III) from Japan on 10 patients with GPP. Clinical benefit of guselkumab was observed already within 1 week in five patients (50%). The treatment efficacy was defined as a Clinical global impression score of ‘very much improved,’ ‘much improved,’ or ‘minimally improved.’ In this study, guselkumab 50 mg was administrated subcutaneously at Weeks 0 and 4, and every 8 weeks until Week 52. At Week 52, the of ‘very much improved’ was achieved in all patients that completed the study (n = 8) (Citation12).
In our case report we successfully treated a patient with severe GPP by targeting the IL-23 pathway with guselkumab. A remission of GPP was observed already after 12 weeks of treatment initiation. The remission was sustained during maintenance treatment with guselkumab in following 18 months. Detraction of corticotherapy did not lead to any recurrence of GPP.
These results support the evidence that guselkumab could provide an effective therapeutic approach in the treatment of GPP.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1–3. doi:10.1111/jdv.14386.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15(9):907–919. doi:10.1080/1744666X.2019.1648209.
- Griffiths CEM, Camp RDR, Barker JNWN. Psoriasis rook’s textbook of dermatology. Oxford: Blackwell Publishing Inc; 2008. p. 1731–1800.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37–42. doi:10.2147/PTT.S181808.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32(10):1645–1651. doi:10.1111/jdv.14949.
- Marrakchi S, Puig L. Pathophysiology of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(Suppl 1):13–19. doi:10.1007/s40257-021-00655-y.
- Morita A, Kotowsky N, Gao R, et al. Patient characteristics and burden of disease in japanese patients with generalized pustular psoriasis: results from the medical data vision claims database. J Dermatol. 2021;48(10):1463–1473. doi:10.1111/1346-8138.16022.
- Bernardo D, Thaçi D, Torres T. Spesolimab for the treatment of generalized pustular psoriasis. Drugs. 2024;84(1):45–58. doi:10.1007/s40265-023-01988-0.
- Krueger J, Puig L, Thaçi D. Treatment options and goals for patients with generalized pusular psoriasis. Am J Clin Dermatol. 2002;23(Suppl 1):51–64. doi:10.1007/s40257-021-00658-9.
- Kamiya K, Ohtsuki M. Epidemiological survey of patients with pustular psoriasis in the japanese society for psoriasis research from 2017 to 2020. J Dermatol. 2023; Jan50(1):3–11. doi:10.1111/1346-8138.16583.
- Lu J, Huang D, Yang N, et al. Better efficacy, lower recurrence rate and decreased CD8 + TRM with guselkumab treatment for generalized pustular psoriasis: a prospective cohort study from China. Clin Immunol. 2024;259:109899. doi:10.1016/j.clim.2024.109899.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45(5):529–539. doi:10.1111/1346-8138.14294.