Abstract
Generalized pustular psoriasis (GPP) in pregnancy can lead to severe complications for both mother and fetus. The treatment of this disease is challenging, especially in recalcitrant and severe cases. Until present, there are no evidence-based guidelines for the treatment of GPP in pregnancy. Spesolimab, a human monoclonal antibody against the IL-36 receptor, has recently attracted attention as a new therapy for GPP flare. This biologic provides rapid and sustained control of symptoms of GPP flare, although its use in pregnant women has not been reported to date. Here, we report a pregnant woman with refractory GPP who did not respond well to systemic steroids. Administration of spesolimab resulted in complete control of the disease and the birth of a healthy baby. Our case demonstrates that IL-36RN inhibitors are a potentially effective and safe treatment option for GPP in pregnancy.
Introduction
Generalized pustular psoriasis (GPP) is a rare, potentially life-threatening neutrophilic dermatosis triggered by stress, corticosteroid withdrawal, pregnancy or infection (Citation1,Citation2). When GPP is associated with pregnancy, it often occurs in the last trimester, resolves after birth, and recurs in subsequent pregnancies (Citation1). Severe and prolonged cases can lead to severe complications in both the mother and fetus, including maternal death, stillbirth and premature delivery (Citation1). The management of GPP during pregnancy is challenging, especially recalcitrant cases. The safety profiles of many drugs used to treat GPP are not fully known (Citation2). As a new therapy for GPP, spesolimab, a human monoclonal antibody against the IL-36 receptor (IL-36R), has recently attracted increased amounts of attention. Here, we describe a pregnant woman with recalcitrant GPP who was successfully treated with spesolimab.
Case report
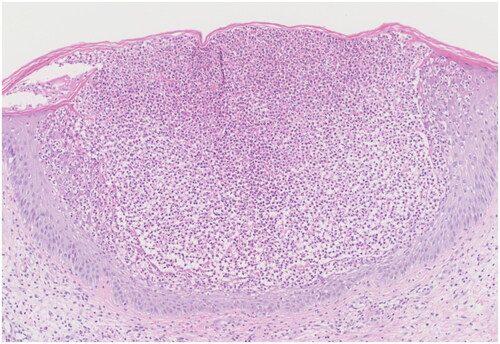
A 24-year-old woman in the 15th week of gestation presented to our clinic with a history of high fever, extensive erythema and pustules for one week. The patient had experienced mild psoriasis vulgaris for seven years, which was well controlled by topical corticosteroids. This was the patient’s first pregnancy. She was otherwise healthy and denied having a family history of similar symptoms. On admission, her body temperature was 38.6 °C. She had fatigue and complained of severe pain. Physical examination revealed generalized edematous erythema with numerous 2- to 3-mm pustules that partially coalesced into pus lakes on the trunk and extremities (). Laboratory examination revealed that the patient had leukocytosis (13,170/μL), neutrophilia (12,510/μL), and an elevated C-reactive protein (CRP) level (55.56 mg/L) and erythrocyte sedimentation rate (ESR) (64 mm/h). A skin biopsy obtained from the patient’s arm revealed that the patient had Kogoj’s spongiform pustules (). The diagnosis of GPP was confirmed.
Figure 1. (A) Generalized edematous erythema with numerous pustules over the body. (B) 48h after application of spesolimab, the lesions were significantly improved.
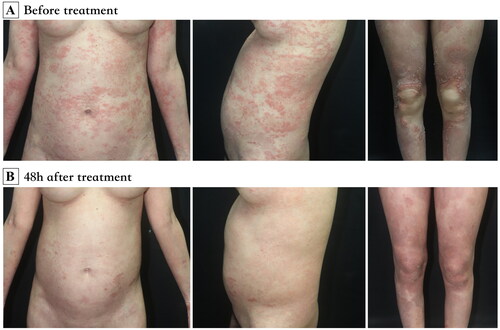
Figure 2. A histopathological examination of a punch biopsy specimen from the arm shows subcorneal pustules with epidermal spongiosis (hematoxylin-eosin; original magnification ×100).
The patient initially received 30 mg prednisone once daily for 10 days, which was then increased to 50 mg once daily due to a lack of response. One week later, her temperature decreased to 37.5 °C, but the rash continued to progress, with the development of numerous new pustules and severe pain. With the patient’s informed consent, 900 mg of spesolimab was administered. All pustules promptly resolved within 48 h (). The neutrophil level, CRP level and ESR all fell within the normal range. Prednisone was gradually decreased (reduced dose by 5 mg every three days until 30 mg and reduced by 2.5 mg every week until 15 mg) and maintained at 15 mg once daily. Three months after receiving spesolimab, the patient reported the occurrence of scattered scaly plaques all over her body but no relapse of edematous erythema or pustules. Topical steroids were administered, but the number of plaque lesions gradually increased. At 38 + 5 gestational weeks, the patient gave birth to a healthy male baby weighing 2800 g vaginally. The steroid was tapered (reduced by 2.5 mg every week until discontinuation). One month later, the patient was administered ixekizumab to control plaque psoriasis.
Discussion
GPP is a rare, potentially life-threatening, neutrophic dermatosis characterized by the sudden eruption of sterile pustules, usually accompanied by systemic symptoms (Citation1). Impetigo herpetiformis (IH) is a subtype of GPP that is induced and exacerbated by pregnancy. GPP commonly occurs in the last trimester of pregnancy and resolves after birth. Recurrence in subsequent pregnancies, with earlier onset and greater severity, is frequent (Citation1). Under severe and long-lasting conditions, IH may lead to severe complications in both the mother and fetus, including maternal death, placental insufficiency, fetal abnormalities, stillbirth and early neonatal death (Citation1).
GPP is known to be associated with the IL-1/IL-36 axis. The proinflammatory ligands IL-36 (α, β, γ) exert intracellular responses through IL-36R, which is expressed on keratinocytes, dendritic cells, macrophages, neutrophils, and T helper cells (Citation3). Activation and upregulation of IL-36R results in a downstream signaling cascade mainly driven by IL-1, IL-6, IL-8 and TNF-α/IL-17A (Citation4). This cytokine storm attracts a neutrophilic infiltrate to the epidermis and leads to neutrophil-driven inflammatory responses (Citation5). Loss-of-function mutations in the IL-36RN have been reported account for 10–82% of patients with GPP; and studies have also demonstrated mutations in other genes with functional connection with the IL-36 pathway, such as CARD14, APS1S3 and SERPINA3 (Citation6). In this case, we performed whole genome sequencing and no mutation was detected in the disease-related genes. The exact mechanisms underlying exacerbation of GPP in pregnancy remains unknown. It has been shown that the IL-36 cytokines are expressed in trophoblast cells and further play a potential role in response to infections and placentation process (Citation7).
Management of this disease is challenging, especially in recalcitrant cases. Currently, evidence-based guidelines for the treatment of pregnant patients with GPP are lacking (Citation2). Topical steroids and narrow-band ultraviolet-B are helpful for patients with mild to moderate GPP. For severe cases, systemic therapies are necessary, and management includes corticosteroids, cyclosporine, granulocyte and monocyte apheresis and biologics. The use of biologics such as TNF-α inhibitors, IL-17 inhibitors and IL-23 inhibitors has been reported in severe refractory cases, although they have not been approved for GPP due to undetermined efficacy and safety profiles in pregnant women (Citation2).
Spesolimab, an anti-IL-36 receptor monoclonal antibody, has been reported to achieve rapid and sustained control of GPP flare symptoms (Citation8). Spesolimab was approved for the treatment of GPP flares by the FDA in 2022. Inhibition of the IL-36 signaling pathway by spesolimab leads to a decreased downstream inflammatory cascade and decreased recruitment of neutrophils and other immune cells (Citation4). Thus, it efficiently prevents the physiological effects of IL-36 cytokines and achieves the purpose of treating GPP (Citation9). Since human immunoglobulin is known to cross the placental barrier, it is preferable to avoid the use of biologics during pregnancy, especially in the third trimester. In this case, spesolimab was used before the third trimester, which is a comparatively safe way to control recalcitrant GPP.
In this case of refractory GPP, we observed a patient with a normal pregnancy, a healthy baby and complete control of the disease in response to treatment with spesolimab. However, further studies are needed, and our findings suggest the potential use of IL-36RN inhibitors as effective and safe treatment options for GPP during pregnancy.
Acknowledgments
We thank the patient for granting permission to publish this information.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Babuna Kobaner G, Polat Ekinci A. Infliximab for the treatment of recalcitrant generalized pustular psoriasis of pregnancy: report of a challenging case. Dermatol Ther. 2020;33(4):e13571. doi: 10.1111/dth.13571.
- Seishima M, Fujii K, Mizutani Y. Generalized pustular psoriasis in pregnancy: current and future treatments. Am J Clin Dermatol. 2022;23(5):661–671. doi: 10.1007/s40257-022-00698-9.
- Murrieta-Coxca JM, Rodríguez-Martínez S, Cancino-Diaz ME, et al. IL-36 cytokines: regulators of inflammatory responses and their emerging role in immunology of reproduction. Int J Mol Sci. 2019;20(7):1649. doi: 10.3390/ijms20071649.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140(1):109–120. doi: 10.1016/j.jaci.2016.08.056.
- Kodali N, Blanchard I, Kunamneni S, et al. Current management of generalized pustular psoriasis. Exp Dermatol. 2023;32(8):1204–1218. doi: 10.1111/exd.14765.
- Baum P, Visvanathan S, Garcet S, et al. Pustular psoriasis: molecular pathways and effects of spesolimab in generalized pustular psoriasis. J Allergy Clin Immunol. 2022;149(4):1402–1412. doi: 10.1016/j.jaci.2021.09.035.
- Murrieta-Coxca JM, Gutiérrez-Samudio RN, El-Shorafa HM, et al. Role of IL-36 cytokines in the regulation of angiogenesis potential of trophoblast cells. Int J Mol Sci. 2020;22(1):285. doi: 10.3390/ijms22010285.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385(26):2431–2440. doi: 10.1056/NEJMoa2111563.
- Iznardo H, Puig L. Exploring the role of IL-36 cytokines as a new target in psoriatic disease. Int J Mol Sci. 2021;22(9):4344. doi: 10.3390/ijms22094344.

