Abstract
In this report, we describe the case of a 28-year-old female with bilateral breast cancer in the setting of a BRCA1 mutation, who presented to dermatology with an eczematous reaction, ultimately diagnosed as a cutaneous immune-related adverse event (cirAE) secondary to an immune checkpoint inhibitor (ICI), pembrolizumab. Our case report highlights a novel therapeutic option for an eczematous cirAE: the topical JAK 1/2 inhibitor, ruxolitinib. CirAEs can occur in up to 55% of patients on ICIs, a class of medications seeing rapidly increasing use in cancer therapy, and prior research has demonstrated that ICI-induced dermatitis may involve different pathways than traditionally observed in their spontaneous counterparts. Specifically, marked Th1 skewing is noted in ICI-induced dermatitis, as opposed to a predominant Th2 response which typically characterizes spontaneous atopic dermatitis. To our knowledge, this is the first case report in the literature discussing use of a topical JAK inhibitor, ruxolitinib, in the treatment of topical steroid-refractory cirAEs. Furthermore, as topical JAK inhibitors are thought to not carry the risks of systemic JAK inhibitors, including malignancy, ruxolitinib cream is a promising therapeutic option for this challenging patient population.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of cancer by inhibiting negative immune regulators, including programmed cell death 1 (PD-1), to upregulate immune responses (Citation1). Various toxicities can arise in patients on ICIs, including cutaneous immune-related adverse events (cirAEs), estimated to affect 14–55% of patients taking ICIs; notably, the presence of cirAEs is associated with improved overall and progression-free survival (Citation1). The majority of cirAEs can be treated with emollients and high-potency topical corticosteroids, but when these fail, other treatment options include oral corticosteroids, phototherapy, or biologic medications (Citation2). An additional class of medications that has shown promise is oral Janus kinase (JAK) inhibitors, which block downstream signaling of multiple cytokines involved in immune-related adverse event development (Citation3). While JAK inhibitors are FDA-approved for eczema, studies have shown that ICI-induced dermatitis may involve different pathways than traditionally observed in their spontaneous counterparts (Citation4). Herein, we report a case of a patient with ICI-induced eczematous dermatitis successfully treated with a topical JAK inhibitor, ruxolitinib 1.5% cream, after trialing topical corticosteroids.
Case report
A 28-year-old BRCA1-positive white female was diagnosed with left triple-negative breast cancer and right breast ductal carcinoma in situ. Treatment was planned to include pembrolizumab every three weeks for 12 cycles concurrently with cyclophosphamide and doxorubicin every two weeks for four cycles, followed by paclitaxel and carboplatin weekly for 12 cycles. When she presented for her third cycle of pembrolizumab, she had a pruritic, erythematous maculopapular rash on her bilateral hands and feet as well as her left elbow with associated skin thickening, which was diagnosed as hand-foot syndrome secondary to doxorubicin by her oncology team (Day 0, initial onset of rash). She reported improvement after using prescribed ammonium lactate 12% cream and fluocinonide 0.05% ointment twice daily for 36 days, after which her rash nearly resolved, with no delays in oncologic therapy.
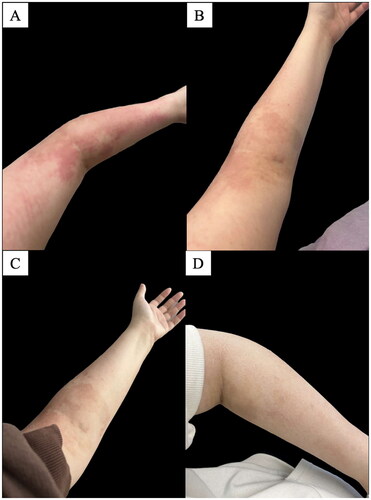
After three additional pembrolizumab infusions, the patient presented to the emergency department with a new pruritic, erythematous rash on her bilateral wrists, bilateral upper extremities, face, and neck, as well as cough. A workup was done to rule out pneumonia for her respiratory symptoms; for the rash, she was given betamethasone dipropionate 0.05% topical ointment (Day 80, since onset of initial rash). Given the new rash, the oncology team switched from paclitaxel (10/12 cycles) to nab-paclitaxel for cycle 11 (Day 85) and 12 while continuing pembrolizumab. The patient’s rash remained after using betamethasone dipropionate 0.05% topical ointment from the emergency department three times total. She was seen by dermatology (Day 91), and the rash appeared as erythematous patches on the patient’s bilateral upper extremities and face (body surface area: 5%), diagnosed as an ICI-induced eczematous rash with an Investigator Global Assessment (IGA) score of 3 (moderate). Given the BSA < 10%, the patient’s cirAE was grade 1 based on the Common Terminology Criteria for Adverse Events (CTCAE). She was prescribed triamcinolone 0.1% lotion twice daily for ten days and hydrocortisone 2.5% cream twice daily for three days followed by once daily for three days. One week later (Day 98), 13 days after cycle 7 of pembrolizumab, after strictly adhering to twice daily use of a class III topical corticosteroid (triamcinolone 0.1% lotion), the rash had not improved. She was advised to discontinue her topical steroids and instead prescribed ruxolitinib 1.5% cream to apply twice daily. The following week, pembrolizumab (cycle 8) was held for one three-week cycle, during which the patient continued using topical ruxolitinib daily, and subsequently experienced near-total rash resolution (). Pembrolizumab treatment was resumed, and the patient remained clear using topical ruxolitinib alone throughout cycles 8 (day 127) and 9 (day 147) of pembrolizumab. She reported applying the topical ruxolitinib on the day of her infusions and once daily for one week thereafter, and as needed if she felt an area of itch, with no recurrence of any rash. She denied the use of any other topical medications or oral antihistamines.
Figure 1. Clinical photos demonstrated resolution of eczematous cirAE throughout treatment with ruxolitinib 1.5% cream on the following days: (A) Day 96; pre-ruxolitinib 1.5% cream, (B) Day 100; day two of ruxolitinib 1.5% cream, (C) Day 104; day six of ruxolitinib 1.5% cream, (D) Day 127; day 29 of ruxolitinib 1.5% cream.
Discussion
Cutaneous adverse reactions to ICI therapy can significantly impact patients’ quality of life during treatment, sometimes leading to delays in immunotherapy administration, as in this case. This patient did not experience symptomatic relief or resolution of her rash after using topical 12% ammonium lactate and three low- to medium-potency topical corticosteroids (fluocinonide, triamcinolone, and hydrocortisone), used for approximately six weeks cumulatively. Two recent case reports revealed efficacy of oral JAK inhibitors for the treatment of recalcitrant ICI-induced cirAEs—oral baricitinib (JAK1/2 inhibitor) treated pyoderma gangrenosum (Citation5) and oral ruxolitinib (JAK1/2 inhibitor) managed erosive penile ulcerations (Citation6).
To our knowledge, this is the first case report demonstrating the use of ruxolitinib cream for treatment of an ICI-induced eczematous cirAE. Reschke et al. have found that Th1 pathway-associated genes, including interferon-gamma (IFN-γ), which signals through the JAK/STAT pathway, are upregulated in inflamed lesional skin in cirAEs; Th1/Tc1 cytokines are predominant in cirAE dermatitis without corresponding elevated expression of Th2 cytokines (Citation4). Atopic dermatitis, for which topical ruxolitinib received FDA approval in 2021, is generally characterized by elevated Th2 activation in both the acute and chronic phase, while Th1 activation is present only in the chronic phase (Citation7). While molecular makeup of atopic dermatitis can vary significantly between patients based on many factors, the marked Th1 skewing demonstrated in lesional skin of ICI-induced dermatitis could potentially be considered distinct from non-ICI-induced atopic dermatitis. The nature of ruxolitinib, through its dual JAK1 and JAK2 inhibition, may allow for targeting of both Th1 and Th2, which may explain its applicability to both spontaneous atopic dermatitis and this acute eczematous cirAE (Citation8). In terms of safety, a prior clinical trial of patients with atopic dermatitis treated with ruxolitinib 1.5% cream showed that even in maximum-use conditions (>25% and <40% body surface area), mean plasma levels of ruxolitinib were much lower than the level required to provoke JAK-mediated myelosuppression (Citation9). Topical JAK inhibition could potentially mitigate the IFN-γ signal transduction involved in cirAE pathogenesis while acting as a non-steroidal therapeutic that does not affect the systemic response to ICIs or cause the JAK inhibitor-associated side effects (malignancy, major adverse cardiovascular events [MACE], opportunistic infections) included in the boxed warnings for all oral and topical JAK inhibitors (Citation10, Citation11). Importantly, a recent meta-analysis of over 20,000 patients across 35 randomized controlled trials showed no increased risk of MACE, all-cause mortality, or venous thromboembolism in patients using oral or topical JAK inhibitors for a dermatologic condition, though they did not investigate risk of malignancy (Citation12).
In this case report, a patient with an ICI-induced grade I eczematous cirAE experienced significant clinical improvement, achieving complete resolution of her rash and relief from itching with the topical JAK inhibitor, ruxolitinib 1.5% cream, following minimal improvement with low- to medium-potency topical corticosteroids. Eczematous cirAEs have been reported to have a wide range of severities; similarly, pruritus, while usually mild or moderate, may also be severe (Citation13). Importantly, the CTCAE grading criteria is based on BSA affected, with or without symptoms, until BSA > 30%; thus, patients with limited skin involvement may be diagnosed with a low-grade cirAE despite symptoms that greatly impact their quality of life. For patients with cirAEs involving a limited BSA (grade I/II) and experiencing disruptive symptoms, topical ruxolitinib could be considered as a treatment option. In particular, patients with severe itch may benefit, as topical ruxolitinib has shown to significantly reduce itch within 12 hours of application (Citation14). This patient reported her itch had disappeared within the first day of application, and she experienced an IGA reduction from 3 (moderate) at her initial visit to 0 (clear) between days 98 and 147, though notably these days are based on appointment times and the patient reported improvement far before clinical follow-up on day 147. Although the pause in pembrolizumab for three weeks could be partially responsible for the initial improvement, the patient continued to remain clear during a rechallenge with the ICI; subsequent cycles of pembrolizumab were tolerated without recurrence of itch or rash, and with no further pauses or adjustments in cancer treatment. The patient experienced no adverse effects with topical ruxolitinib. The promising results of this case suggest that larger, prospective studies are warranted to confirm whether topical ruxolitinib may represent a safe and effective non-steroidal therapeutic for ICI-induced eczematous cirAEs.
Patient consent
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Reprint requests
Nicholas Gulati.
Acknowledgments
We acknowledge the Tisch Cancer Institute’s Cancer Clinical Investigation program, an NIH center: project number 5P30CA196521-09.
Disclosure statement
The authors report there are no competing interests to declare.
References
- Nikolaou V, Tsimpidakis A, Stratigos A. Cutaneous adverse reactions of immunotherapy in patients with advanced melanoma. Cancers (Basel). Mar 31 2023;15(7):2084. doi: 10.3390/cancers15072084.
- Phillips GS, Wu J, Hellmann MD, et al. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol. Oct 20 2019;37(30):2746–2758. doi: 10.1200/JCO.18.02141.
- Henderson Berg M-H, Del Rincón SV, Miller WH. Potential therapies for immune-related adverse events associated with immune checkpoint inhibition: from monoclonal antibodies to kinase inhibition. J Immunother Cancer. Jan 2022;10(1):e003551. doi: 10.1136/jitc-2021-003551.
- Reschke R, Shapiro JW, Yu J, et al. Checkpoint blockade-induced dermatitis and colitis are dominated by tissue-resident memory T cells and Th1/Tc1 cytokines. Cancer Immunol Res. Oct 4 2022;10(10):1167–1174. doi: 10.1158/2326-6066.CIR-22-0362.
- Kim HS, Kwon JE, Park YJ. Atezolizumab plus bevacizumab-induced recalcitrant pyoderma gangrenosum treated with baricitinib: a case report. Acta Derm Venereol. Aug 1 2023;103:adv9646. doi: 10.2340/actadv.v103.9646.
- Chen CY, Chiu CF, Bai LY. Treatment of pembrolizumab-induced cutaneous lesions with ruxolitinib. Eur J Cancer. May 2019;113:69–71. doi: 10.1016/j.ejca.2019.03.016.
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. Apr 2017;139(4S):S65–S76. doi: 10.1016/j.jaci.2017.01.011.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. Jun 21 2017;15(1):23. doi: 10.1186/s12964-017-0177-y.
- Bissonnette R, Call RS, Raoof T, et al. A maximum-use trial of ruxolitinib cream in adolescents and adults with atopic dermatitis. Am J Clin Dermatol. May 2022;23(3):355–364. doi: 10.1007/s40257-022-00690-3.
- Reschke R, Gajewski TF. Tissue-resident memory T cells in immune-related adverse events: friend or foe? Oncoimmunology. 2023;12(1):2197358. doi: 10.1080/2162402X.2023.2197358.
- Ytterberg SR, Bhatt DL, Connell CA. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. Reply. N Engl J Med. May 5 2022;386(18):1768–1326. doi: 10.1056/NEJMc2202778.
- Ingrassia JP, Maqsood MH, Gelfand JM, et al. Cardiovascular and venous thromboembolic risk with JAK inhibitors in immune-mediated inflammatory skin diseases: a systematic review and Meta-Analysis. JAMA Dermatol. 2023;160(1):28–36. Nov 1 doi: 10.1001/jamadermatol.2023.4090.
- Chen CH, Yu HS, Yu S. Cutaneous adverse events associated with immune checkpoint inhibitors: a review article. Curr Oncol. Apr 18 2022;29(4):2871–2886. doi: 10.3390/curroncol29040234.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. Oct 2021;85(4):863–872. doi: 10.1016/j.jaad.2021.04.085.

