 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Brodalumab, a human monoclonal antibody that targets interleukin-17 receptor A (IL-17RA), is approved in the US and EU for treatment of adults with moderate-to-severe plaque psoriasis. Although brodalumab has demonstrated efficacy and safety vs placebo in clinical trials of patients with psoriasis and psoriatic arthritis (PsA), real-world evidence is needed to evaluate long-term effectiveness and safety of brodalumab in routine care. This interim analysis of the German Psoriasis Registry PsoBest examined patient profiles, treatment outcomes, and drug survival of first-time use of brodalumab for 12 months in adult patients with moderate-to-severe plaque-type psoriasis (with and without PsA) (data cutoff: June 30, 2021). Clinician and patient-reported outcomes of the total cohort (n = 227; PsA, n = 38) indicated a rapid response to brodalumab treatment within the first 3 months, which was maintained up to 12 months. The overall one-year drug survival rate was 76.2%, the mean time to discontinuation was 8.3 months. Reasons for discontinuation were mainly loss/lack of effectiveness, followed by adverse events, contraindication and skin clearance. In sum, brodalumab demonstrated rapid and sustained effectiveness and was well-tolerated over 12 months in German patients with moderate-to-severe psoriasis and PsA in a real-world setting.
1. Introduction
Psoriasis is an immune-mediated chronic inflammatory disease that affects 2-4% of the population in western countries (Citation1, Citation2). The most common disease variant is plaque psoriasis (about 85% of cases), which typically manifests as erythematous plaques with thick scaling on limbs, trunk, and scalp (Citation3). Approximately 30% of patients with psoriasis develop psoriatic arthritis (PsA), an inflammatory arthritis characterized by asymmetric oligoarthritis, enthesitis, and/or dactylitis (Citation4–6). Individuals with psoriasis show substantial comorbidity with other diseases (e.g., cardiovascular and psychiatric disease) starting even in childhood (Citation7) and a high burden of disease that negatively impacts patient quality of life (QoL) and life course (Citation8, Citation9).
Psoriasis pathogenesis involves chronic activation of the interleukin-23/interleukin-17 (IL-23/IL-17) signaling pathway (Citation10). Brodalumab is a monoclonal antibody approved in the United States and European Union for the treatment of adults with moderate-to-severe plaque psoriasis (Citation11, Citation12). It is the first biological therapy that specifically targets the subunit A of the IL-17 receptor, inhibiting downstream signaling of multiple IL-17 family cytokines which may contribute to psoriasis pathogenesis (Citation13). In phase 3 clinical trials, brodalumab-treated patients with moderate-to-severe psoriasis achieved high levels of skin clearance for up to 52 weeks (Citation14, Citation15), with longer sustained response and greater cumulative clinical treatment benefits versus ustekinumab (Citation16, Citation17). Pooled data from the phase 3 AMAGINE-2 and −3 trials showed that brodalumab was well-tolerated and resulted in high levels of skin clearance that were rapidly achieved and maintained through Week 120 (Citation18). Additionally, brodalumab has demonstrated efficacy in psoriasis patients with PsA in both phase 2 and 3 clinical trials (Citation19, Citation20).
Recent real-world data in patients from Italy (Citation21,Citation22) and Greece (Citation23–25) showed brodalumab led to rapid and sustained improvements in symptoms and QoL with a favorable safety profile. However, a more comprehensive evaluation of the long-term ability of brodalumab to maintain effectiveness and safety in routine clinical practice in psoriasis patients with vs without PsA is needed. Here, we present interim descriptions of patient profiles, treatment outcomes, and drug survival of brodalumab in psoriasis patients, with and without PsA, from the German Psoriasis Registry PsoBest.
2. Methods
2.1. Patient population
The German Psoriasis Registry PsoBest documents the long-term course of moderate-to-severe psoriasis (Citation26) in patients initiating systemic treatment (non-biologics, biologics including biosimilars) for the first time. Patient observation time is up to ten years, regardless of subsequent treatment course. Follow-up visits in clinical settings are conducted at intervals of 3 months in the first half-year and every 6 months thereafter. Clinical outcomes, patient benefits, treatment regimens, and drug safety are measured and reported on a regular basis (Citation27).
The registry includes adult patients (≥18 years) with moderate-to-severe plaque-type psoriasis, with and without PsA; patients diagnosed with pure forms of inverse or pustular psoriasis are not included. To minimize selection bias for 12-month drug survival due to early censored cases in ongoing data collection, only patients who had the chance of being observed for at least 12 months were selected (registry inclusion before June 30, 2020) from the quality-assured PsoBest dataset with a data cutoff of June 30, 2021 (Figure S1). Selection of all patients who initiated brodalumab treatment (dosing of 210 mg every two weeks) for the first time resulted in an analysis set of 227 patients. PsA involvement was determined by an algorithm developed in cooperation with rheumatologists published elsewhere (Citation5).
2.2. Comorbidity and comedication
Data on comorbidity and comedication at baseline was assessed using a list of predefined conditions and diagnoses with limited verbatim additions (Methods S1). The existence of comorbidity was assumed if medication in the treatment of a comorbidity was utilized, and verbatim diagnoses were assigned to comorbidity categories. With direct questioning for relevant diagnoses and the possibility of adding limited verbatim text, we assume that overestimation by suspected comorbidity is improbable.
2.3. Clinician- and patient-reported outcomes
The Psoriasis Area and Severity Index (PASI) (Citation28, Citation29) was used for the assessment of clinical response, which was defined as achieving 75%, 90%, or 100% improvement in PASI relative to baseline (PASI-75/90/100). For PsA, a 10-point visual analogue scale (VAS) was utilized to determine PsA severity. Affected joints were recorded by the dermatologist, and the number of swollen and painful joints was calculated.
Patient-reported outcomes (PROs) included scores from two validated patient-reported questionnaires, including the Dermatology Life Quality Index (DLQI) (Citation30, Citation31) and Patient Benefit Index (PBI) (Citation32). The proportion of patients who achieved a DLQI score between 0 and 1 (indicating no impact on patient’s life), a PBI score of at least 1 (indicating a minimum clinically-relevant benefit), and a PBI score between 3 and 4 (indicating a high clinically-relevant benefit) were calculated.
Since routine health care in a real-world setting does not exactly follow registry design, the dates of dermatology visits usually differ from the planned dates of measurement. All parameters were evaluated at baseline and visits 2 (3 ± 1 months), 3 (6 ± 1 months), and 4 (12 ± 2 months). Treatment start was allowed 14 days prior to and up to 4 weeks after the baseline visit. All visits between initiation and termination of brodalumab treatment were included in the analyses, regardless of the completeness of visits for each individual patient across time.
2.4. Treatment discontinuation and 12-month drug survival
Treatment phase was defined as the time between initiation and first stop of therapy. Treatment discontinuation was defined as an observed stop of the first treatment phase with no restart of treatment within the following 90 days, which could include censoring, i.e. a “not yet”-observation (ongoing data collection) resulting in “no stop observed” at the date of the last visit reported or at data cutoff. Reasons for treatment discontinuation were categorized as skin clearance, adverse events (AEs), onset of contraindication, lack/loss of effectiveness, other, and unknown. To gain insight into the nature of AEs that resulted in treatment discontinuation, MedDRA® (MedDRA® trademark is registered by ICH) Preferred Term (Citation33), System Organ Class, severity rating, treatment days to event, recovery, and causality are listed.
To minimize bias in interim drug survival analysis (Citation34), the drug survival window was restricted to one-year drug survival and only patients having the chance of a one-year observation were selected (i.e. registered one year before data cutoff) (Citation34). Treatment time for patients observed and on treatment for more than one year (i.e. censored at the end of the drug survival interval) was set to 12.01 months, which was necessary for correct estimation of patients at risk at exactly 12 months.
2.5. Statistical analysis
For comparison of the psoriasis only (Pso) and PsA subgroups, chi-squared tests were performed on categorical variables and t-tests on continuous variables. For comparison of comorbidity/comedication, a stepwise Fisher’s Exact test strategy was applied: Main categories of conditions were compared with presence and medication. Subsumed conditions were compared to identify specific differences in comorbidity only if a significant difference was detected for the main category.
For drug survival, Kaplan-Meier curves were generated, and a log-rank test was performed to compare drug survival times between the two subgroups. The significance level was set at α ≤ 0.05 for all tests. Analyses were performed using SPSS v. 26 (IBM, NY, USA) and figures were generated using GraphPad Prism 9 (GraphPad Software Inc., MA, USA).
3. Results
3.1. Baseline
3.1.1. Patient characteristics
The analysis included a subset of patients from the full PsoBest registry dataset (N = 12,975) with a data cutoff of June 30, 2021. In total, 227 patients were eligible for the present analyses (Figure S1). For the total cohort (N = 227), patients were, on average, middle-aged (mean: 49.9 years), predominately male (69.2%), and had a mean disease duration of 22.0 years (n = 211). Of the total cohort, 55.1% received previous systemic treatment but no prior biologic therapy, and 31.7% received another biologic therapy prior to switching to brodalumab. Mean values in the total cohort for PASI, percent body surface area (BSA), and DLQI were 18.0 (n = 224), 29.6% (n = 214), and 12.5 (n = 220), respectively ().
Table 1. Patient characteristics at baseline.
Of the total cohort, 83% (189/227) had psoriasis (of the skin, Pso) and no psoriatic arthritis and 17% (38/227) had both psoriasis and psoriatic arthritis (PsA). Patient characteristics at baseline were similar between the Pso and PsA subgroups; the only statistically significant difference was a greater mean disease duration in patients with PsA (Mean: 28.3; SD: 18.0) vs Pso (Mean: 20.6; SD: 14.9), p ≤ 0.007 (). The PsA subgroup had a numerically higher mean age (Pso: 49.1 vs PsA: 53.8 years), the proportion of female patients (Pso: 29.1% vs PsA: 39.5%), and the proportion of patients who had received another biologic therapy before switching to brodalumab (Pso: 30.2% vs PsA: 39.5%) ().
3.1.2. Comorbidity and comedication
In the total cohort, the most frequent comorbidity at baseline was cardiovascular disease (34.4%, n = 78/227), followed by psychiatric, sleep and addictive disorder (25.1%, n = 57/227), and metabolic disease (18.5%, n = 42/227) (Table S1). The presence of arthritis (rheumatoid or psoriatic) was the only relevant statistically significant difference in comorbidity/comedication between the Pso and PsA subgroups (Table S2).
3.2. Clinician-assessed outcomes
In the total cohort, mean PASI decreased from baseline (18.0, n = 224) to 12 months (2.3, n = 68) (). Likewise, mean BSA decreased from baseline (29.6, n = 214) to 12 months (3.1, n = 67) (). PASI-75, PASI-90, and PASI-100 achievement increased from 3 months to 12 months: PASI-75 increased from 75.6% to 85.3% (), PASI-90 increased from 58.8% to 76.5% (), and PASI-100 increased from 37.0% to 51.5% ().
Figure 1. Mean (a) PASI (0-72) and (b) BSA (0-100) scores at 3, 6, and 12 months.
Error bars depict standard error. The number of patients at each time point are represented within each respective bar.
BSA, body surface area (range 0-100); PASI, Psoriasis Area and Severity Index (range 0-72); PsA, psoriatic arthritis; Pso, psoriasis; SE, standard error.
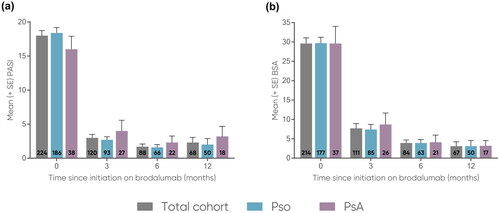
Figure 2. Proportions of patients achieving (a) PASI-75, (b) PASI-90, and (c) PASI-100 at 3, 6, and 12 months.
The number of patients at each time point are represented within each respective bar.
PASI, Psoriasis Area and Severity Index (range 0-72); PASI-75, at least 75% reduction from baseline PASI; PASI-90, at least 90% reduction from baseline PASI; PASI-100, 100% reduction from baseline PASI; PsA, psoriatic arthritis; Pso, psoriasis.
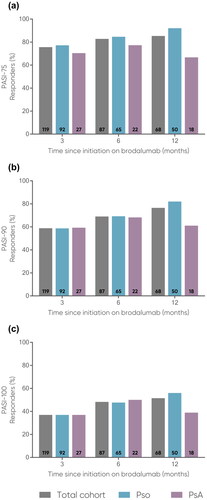
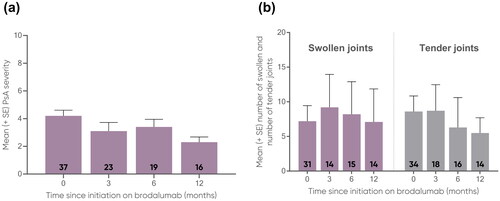
Mean PASI was comparable for both subgroups and decreased from baseline to 12 months (Pso: 18.4 to 2.0; PsA: 16.0 to 3.2) (). Likewise, mean BSA was comparable for both subgroups and decreased from baseline to 12 months (Pso: 29.7 to 3.1; PsA: 29.6 to 3.2) (). PASI-75 achievement increased for the Pso subgroup from 3 months to 12 months (77.2% to 92.0%) and decreased for the PsA subgroup from 3 months to 12 months (70.4% to 66.7%) (). For both subgroups, PASI-90 and PASI-100 achievement increased from 3 months to 12 months. In the Pso subgroup, PASI-90 increased from 58.7% to 82.0% () and PASI-100 increased from 37.0% to 56.0% (). In the PsA subgroup, PASI-90 increased from 59.3% to 61.1% () and PASI-100 increased from 37.0% to 38.9% (). For the PsA subgroup, the largest effectiveness was observed at 6 months for PASI-75 (77.3%, n = 17/22), PASI-90 (68.2%, n = 15/22) and PASI-100 (50.0%, n = 11/22). The mean PsA severity using the VAS 0-10 decreased from 4.2 at baseline (n = 37) to 2.3 at 12 months (n = 16) (), while the mean number of swollen joints decreased from 7.2 at baseline (n = 31) to 7.1 at 12 months (n = 14), and the mean number of tender joints decreased from 8.6 (n = 34) to 5.5 (n = 14), respectively ().
Figure 3. Mean (a) PsA severity (VAS 0-10) and (b) number of swollen (0-74) and number of tender (0-76) joints in PsA patients at 0, 3, 6, and 12 months.
Error bars depict standard error. The number of patients at each time point are represented within each respective bar.
PsA, psoriatic arthritis; SE, standard error; VAS, Visual Analogue Scale.
3.3. Patient-reported outcomes
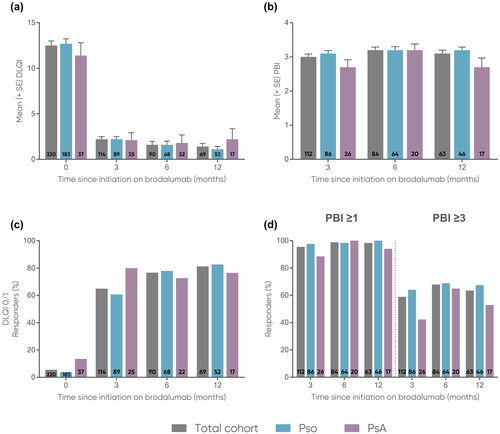
Mean DLQI decreased from baseline to 12 months in the total cohort and across the subgroups (total: 12.5 to 1.4; Pso: 12.7 to 1.1; PsA: 11.4 to 2.2) (). Mean PBI of approximately 3 was maintained from 3 months (total: 3.0; Pso: 3.1, PsA: 2.7) to 12 months (total: 3.1; Pso: 3.2; PsA: 2.7) (). DLQI 0/1 achievement increased from baseline to 12 months in the total cohort and across the subgroups (total: 5.5% to 81.2%; Pso: 3.8% to 82.7%; PsA: 13.5% to 76.5%) (). Achievement of PBI ≥1 increased from 3 months (total: 95.5%; Pso: 97.7%; PsA: 88.5%) to 12 months (total: 98.4%; Pso: 100.0%; PsA: 94.1%) (). PBI ≥3 achievement increased overall from 3 months (total: 58.9%; Pso: 64.0%; PsA: 42.3%) to 12 months (total: 63.5%; Pso: 67.4%; PsA: 52.9%) (). For the PsA subgroup, the largest decrease in mean DLQI (1.8, n = 22), mean increase in PBI (3.2, n = 20), PBI ≥1 achievement (100.0%, n = 20/20), and PBI ≥3 achievement (65.0%, n = 13/20) was observed at 6 months, and largest achievement of DLQI 0/1 occurred at 3 months (80.0%, n = 20/25).
Figure 4. Mean (a) DLQI (0-30) and (b) PBI (0-4) and proportions of patients achieving (c) DLQI 0/1, (d) PBI 1 and PBI
3 at 0, 3, 6, and 12 months.
The number of patients at each time point are represented within each respective bar. For mean DLQI and PBI, error bars depict standard error.
DLQI, Dermatology Life Quality Index; PBI, Patient Benefit Index; PsA, psoriatic arthritis; Pso, psoriasis; SE, standard error.
3.4. Reasons for discontinuation and 12 month drug survival
3.4.1. Treatment discontinuation
Within the whole observational period, 18.1% (41/227) of total patients discontinued treatment (Pso: 18.0%, n = 34/189; PsA: 18.4%, n = 7/38). The average time on brodalumab to discontinuation (cases with a reported stop) in months was 8.3 for the total cohort, 8.0 for the Pso subgroup, and 9.7 for the PsA subgroup. Patients with PsA showed a numerically longer median time to discontinuation (Pso: 5.8 vs PsA: 9.1 months) and narrower time range to discontinuation compared to patients with Pso (Pso: 1.2 to 34.5 months vs PsA: 1.9 to 25.5 months) ().
Table 2. Time to discontinuation (for cases with reported stop).
The primary reason for discontinuation was lack or loss of effectiveness (total: 47.7%; Pso: 41.7%; PsA: 75.0%). The second most common reason for discontinuation were AEs (total: 27.3%; Pso: 30.6%; PsA: 12.5%). Skin clearance was reported twice (both instances in the Pso subgroup) as the reason for treatment discontinuation ().
Table 3. Reasons for discontinuation of brodalumab.
3.4.2. Adverse events leading to discontinuation
Further examination of the AEs reported showed that 55.0% (11/20) of the events were non severe and mostly involved the skin and subcutaneous tissue (4 non severe, 2 severe events in 3 cases). The single observed AE in the PsA subgroup was non severe erythema, accompanied by rash and pruritus of the skin beginning at Day 74 after treatment initiation (). Four of seven serious AEs (57.1%) involved severe infections, including bacterial arthritis (80 days after treatment start; with causality rated as related to treatment) in connection with palmoplantar pustulosis and pyoderma gangrenosum (35 days after treatment start; with causality rated as related to treatment), infective episcleritis (4 days after treatment start; with causality rated as possibly related to treatment), herpes zoster meningitis and ophthalmic herpes infections (350 days after treatment start; with causality rated as possibly related to treatment). Lastly, a joint operation due to preexisting osteoarthritis issues occurred 285 days after treatment start, but causality to treatment was rated as improbable ().
Table 4. Adverse events as reason for discontinuation.
3.4.3. Drug survival
For the total cohort, the mean drug survival rate decreased across the 1-year period of analysis from 96.6% after 3 months, to 86.4% after 6 months, and 76.2% after 12 months. As the drug survival rate remained ≥50% after 12 months, the median survival time was not estimable. There was no statistically significant difference in the estimated mean 12 months survival time (mean time until discontinuation) between the subgroups (Pso: 10.6 months vs PsA: 10.8 months; p ≤ 0.54). Mean 12 months drug survival rates decreased to 97.3%, 85.8%, and 74.9% for the Pso subgroup, and 92.9%, 89.1%, and 83.2% for the PsA subgroup (, ). Of note, the restriction of longer treatment times to 12.01 months resulted in the observation period being censored for 85.9% (195/227) patients of the total cohort (Pso: 85.2%, n = 161/189; PsA: 89.5%, n = 34/38).
Figure 5. Time on brodalumab over 12 months.
Tick marks on connecting lines represent incidences/time points of censoring (i.e. last time of observation, not treatment stop).
PsA, psoriatic arthritis; Pso, psoriasis.
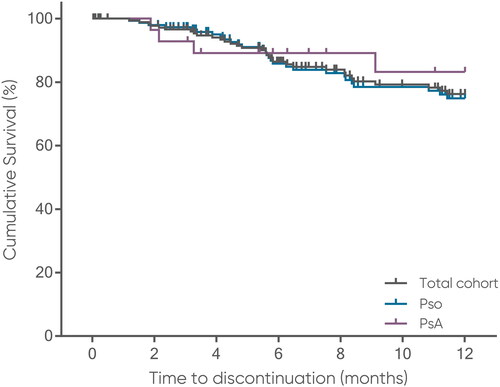
Table 5. Drug survival rate.
4. Discussion
These data reflect, to our knowledge, the largest real-world population analysis of the effectiveness and safety of brodalumab in patients with moderate-to-severe psoriasis. Mean PASI and BSA values indicated that patients receiving brodalumab had moderate-to-severe psoriasis with a high impact on their health-related QoL at baseline. Clinician-assessed outcomes and PROs indicated a rapid response to brodalumab treatment within the first 3 months, which was maintained up to 12 months. Although patients in the PsA subgroup showed slightly worse mean PASI and BSA decline, these differences were not statistically significant at all time points. These real-world data are aligned with long-term efficacy data from clinical trials (Citation35) and real-world effectiveness data from Italy (Citation22) and Greece (Citation24, Citation25). Moreover, the data presented here are similar to a recent post hoc analysis of the phase 3 AMAGINE-2 and AMAGINE-3 trials which showed a comparable degree of brodalumab effectiveness and impact on QoL through 52 weeks in patients with moderate-to-severe psoriasis with and without concomitant PsA (Citation36).
Approximately one-third of patients (Pso: 30.2% vs PsA: 39.5%) had received another biologic therapy prior to brodalumab. Since previous work suggests treatment efficacy is less likely to improve for subsequently offered biologics (Citation37), it is possible that response rates would be lower in a population with a higher proportion of biologic-experienced patients. However, an analysis of the phase 3 AMAGINE-2 and −3 trials demonstrated that rates of skin clearance with brodalumab were similar in biologic-naïve and biologic-experienced patients (Citation38), which aligns with these data, demonstrating that brodalumab is also effective in moderate-to-severe psoriasis patients who did not respond to a previous anti-IL-17 therapy, a finding which may better inform clinical decisions regarding when to modify biologics treatment.
As drug survival is a marker for treatment sustainability in chronic diseases such as psoriasis (Citation39), an overall one-year drug survival rate of approximately 75% and a mean time to discontinuation of 8.2 months indicates high sustainability. In the first 12 months of treatment, there was no difference in average drug survival rate and time between the Pso and PsA subgroups. In both subgroups, the primary reason for treatment discontinuation was a reported lack or loss of effectiveness and the majority of reported AEs were non severe.
The analysis performed was limited by several factors related to its observational and non-interventional design. A lower degree of internal validity is expected in non-interventional studies (compared to clinical trials) as well as a potential for selection bias. These analyses were performed while the registry is ongoing; therefore, the results are interim and all analyses are limited by completeness of the data itself. There is likely only a limited number of patients able to be included at each assessed time point in a long-term analysis, a limitation which may increase the possibility that small differences and rare events could be missed. Moreover, the 12-month follow-up time was shorter when compared to two real-world brodalumab data studies from Greece (Citation24, Citation25), which had follow-up time of 24 months. Lastly, future studies should aim to directly compare data from other current biologics within the same registry.
The analysis and data presented also demonstrates the strength of real-world data collection in patient registries. The protocol-driven data collection of relevant disease and treatment characteristics over a long time-period across a defined geographical region/health care system enables detailed analysis and generalizable data on course of disease, treatment effectiveness, safety, pathways as well as patient benefit, cost of illness and many more relevant dimensions of health care. The evidence generated is an enrichment of scientific medical knowledge useful for medical decisions and thus directly translates into routine health care.
Overall, brodalumab demonstrated rapid and sustained effectiveness and was well-tolerated over 12 months of treatment in German patients with moderate-to-severe psoriasis and psoriatic arthritis in a real-world setting.
Ethical approval
Patients gave written informed consent on registry participation and approval from the local ethics committee was obtained to conduct the PsoBest registry.
Supplemental Material
Download MS Word (52.3 KB)Acknowledgements
The authors thank the Scientific Communication Team of the IVDP, in particular, Amber Hönning and Sara Tiedemann for copy editing.
Disclosure statement
Lisa Schaeffer, Christina Sorbe, and Stephan Jeff Rustenbach are employees at the University Medical Center Hamburg-Eppendorf (UKE) and report no conflicts of interest.
Nesrine Ben-Anaya has served as consultant and/or paid speaker of the following companies: AbbVie, Almirall, Incyte, Novartis, UCB, Pfizer.
Ulrich Mrowietz has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Aditxt, Almirall, Amgen, Aristea, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Dr. Reddy’s, Eli Lilly, Formycon, Immunic, Janssen-Cilag, LEO Pharma, Merck, Sharp & Dohme, MetrioPharm, Novartis, Phi-Stone, Sanofi-Aventis, UCB Pharma, UNION therapeutics.
Matthias Augustin has served as consultant and/or paid speaker for and/or has received research grants and/or honoraries for consulting and/or scientific lectures for and/or got travel expenses reimbursed and/or participated in clinical trials sponsored by companies that manufacture drugs including Abbott/AbbVie, ALK Scherax, Almirall, Amgen, Beiersdorf, Biogen Idec, BMS, Boehringer Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Forward Pharma, Fresenius, Galderma, GSK, Hexal, Incyte, Janssen-Cilag, LEO Pharma, Lilly, Medac, Menlo, Merck, MSD, Mylon, Novartis, Pfizer, Regeneron, Sandoz, Sanofi-Aventis, Stallergenes, Stiefel, Teva, TK, Trevi, UCB and Xenoport outside the submitted work.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Nesrine Ben-Anaya upon reasonable request.
Additional information
Funding
References
- Augustin M, Reich K, Glaeske G, et al. Co-morbidity and age-related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venereol. 2010;90(2):1–10. doi: 10.2340/00015555-0770.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595.
- Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):569–579. doi: 10.1016/j.rdc.2015.07.003.
- Langenbruch A, Radtke MA, Krensel M, et al. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br J Dermatol. 2014;171(5):1123–1128. doi: 10.1111/bjd.13272.
- Reich K, Krüger K, Mössner R, et al. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol. 2009;160(5):1040–1047. doi: 10.1111/j.1365-2133.2008.09023.x.
- Augustin M, Glaeske G, Radtke MA, et al. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633–636. doi: 10.1111/j.1365-2133.2009.09593.x.
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2(1):16082. doi: 10.1038/nrdp.2016.82.
- Kimball AB, Gieler U, Linder D, et al. Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol. 2010;24(9):989–1004. doi: 10.1111/j.1468-3083.2010.03705.x.
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–1613. doi: 10.4049/jimmunol.1800013.
- European Medicines Agency. Kyntheum summary of product characteristics. 2017. (cited 2023 Sep12). Available from: https://www.ema.europa.eu/documents/product-information/kyntheum-epar-product-information_en.pdf.
- Valeant Pharmaceuticals Inc. Prescribing information: SILIQTM (brodalumab) injection for subcutaneous use. 2017. (cited 2023 Sep12). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828–3836. doi: 10.4049/jimmunol.1301737.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi: 10.1111/bjd.14493.
- Reich K, Hansen JB, Puig L, et al. Complete clearance and psoriasis area and severity index response for brodalumab and ustekinumab by previous treatment history in AMAGINE-2 and AMAGINE-3. J Eur Acad Dermatol Venereol. 2021;35(10):2034–2044. doi: 10.1111/jdv.17433.
- Warren RB, Hansen JB, Reich K, et al. Complete clearance and psoriasis area and severity index response for brodalumab and ustekinumab in AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2021;35(2):450–457. doi: 10.1111/jdv.16816.
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36(8):1275–1283. doi: 10.1111/jdv.18068.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306. doi: 10.1056/NEJMoa1315231.
- Mease PJ, Helliwell PS, Hjuler KF, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80(2):185–193. doi: 10.1136/annrheumdis-2019-216835.
- Fargnoli MC, Esposito M, Dapavo P, et al. Brodalumab for the treatment of moderate-to-severe plaque-type psoriasis: a real-life, retrospective 24-week experience. J Eur Acad Dermatol Venereol. 2021;35(3):693–700. doi: 10.1111/jdv.16931.
- Galluzzo M, Caldarola G, De Simone C, et al. Use of brodalumab for the treatment of chronic plaque psoriasis: a one-year real-life study in the Lazio region, Italy. Expert Opin Biol Ther. 2021;21(9):1299–1310. doi: 10.1080/14712598.2021.1941862.
- Rigopoulos D, Angelakopoulos C, Apalla Z, et al. Real world experience of brodalumab treatment in patients with moderate-to-severe plaque psoriasis in the Greek population: results from an interim analysis of the BrIDGE study. Dermatol Ther. 2022;35(12):e15886. doi: 10.1111/dth.15886.
- Tampouratzi E, Papakonstantis M, Katsantonis J, et al. Clinical evidence on the use of brodalumab for the treatment of psoriasis in Greece: experience from clinical practice of four tertiary hospitals. Dermatol Ther. 2022;35:e15532.
- Papadavid E, Zafeiriou E, Georgiou S, et al. Real-world clinical outcomes of treatment with brodalumab in patients with moderate-to-severe psoriasis: a retrospective, 24-month experience from four academic dermatology centers in Greece. J Dermatolog Treat. 2022;33(7):3053–3059. doi: 10.1080/09546634.2022.2110836.
- Augustin M, Spehr C, Radtke MA, et al. German psoriasis registry PsoBest: objectives, methodology and baseline data. J Dtsch Dermatol Ges. 2014;12(1):48–57. doi: 10.1111/ddg.12233.
- Reich K, Mrowietz U, Radtke MA, et al. Drug safety of systemic treatments for psoriasis: results from the German psoriasis registry PsoBest. Arch Dermatol Res. 2015;307(10):875–883. doi: 10.1007/s00403-015-1593-8.
- Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839.
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210(3):194–199. doi: 10.1159/000083509.
- Basra MK, Fenech R, Gatt RM, et al. The dermatology life quality index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035. doi: 10.1111/j.1365-2133.2008.08832.x.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x.
- Augustin M, Radtke MA, Zschocke I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res. 2009;301(8):561–571. doi: 10.1007/s00403-009-0928-8.
- ICH. Medical Dictionary for Regulatory Activities: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. (cited 2023 Sep 14 September). Available from: https://www.ich.org/page/meddra.
- van Houwelingen HC, van de Velde CJ, Stijnen T. Interim analysis on survival data: its potential bias and how to repair it. Stat Med. 2005;24(18):2823–2835. doi: 10.1002/sim.2248.
- Lebwohl MG, Blauvelt A, Menter A, et al. Efficacy, safety, and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis treated with brodalumab for 5 years in a long-term, Open-Label, phase II study. Am J Clin Dermatol. 2019;20(6):863–871. doi: 10.1007/s40257-019-00466-2.
- Kokolakis G, Vadstrup K, Hansen JB, et al. Brodalumab is associated with high rates of complete clearance and quality of life improvement: a subgroup analysis of patients with psoriasis and concomitant psoriatic arthritis. Dermatology. 2022;238(4):620–629. doi: 10.1159/000520290.
- Karczewski J, Poniedziałek B, Rzymski P, et al. Factors affecting response to biologic treatment in psoriasis. Dermatol Ther. 2014;27(6):323–330. doi: 10.1111/dth.12160.
- Papp KA, Gordon KB, Langley RG, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3. Br J Dermatol. 2018;179(2):320–328. doi: 10.1111/bjd.16464.
- Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016;30(7):1148–1158. doi: 10.1111/jdv.13611.

