Abstract
In the KEEPsAKE 1 (NCT03675308) and KEEPsAKE 2 (NCT03671148) phase 3 trials, risankizumab demonstrated greater efficacy compared with placebo in patients with active psoriatic arthritis (PsA). This post hoc integrated analysis evaluated achieving the following efficacy outcomes at weeks 24 and 52 by baseline demographics and clinical characteristics: ≥20%/50%/70% improvement in American College of Rheumatology response criteria (ACR20/50/70), ≥90% improvement in Psoriasis Area and Severity Index, minimal disease activity status, Low Disease Activity status (Disease Activity in Psoriatic Arthritis), and minimal clinically important difference in pain. Baseline demographics and clinical characteristics were similar between risankizumab (n = 707) and placebo (n = 700) groups. Numerically higher ACR20 response rates at week 24 (primary endpoint) were observed among the risankizumab (46.3%−60.1%) vs. placebo (15.5%−36.2%) cohorts, regardless of subgroups. At week 52, consistent proportions of patients randomized to risankizumab achieved ACR20 (48.6%−75.8%) while those initially randomized to placebo and switched to risankizumab experienced an improvement from week 24 (43.7%−63.9%), regardless of subgroups. Similar trends were observed for other efficacy measures assessing rigorous skin response criteria, composite measures of overall disease activity, and PsA-related symptoms. Risankizumab treatment was efficacious among patients with varying demographic and psoriatic disease characteristics through 52 weeks.
Introduction
Psoriatic arthritis (PsA) is a progressive, chronic, inflammatory disease characterized by the association of psoriasis and musculoskeletal inflammation (Citation1,Citation2). While recent scientific advances have led to efficacious treatments for active PsA, patients with other comorbidities (e.g., obesity) or prior exposure to biologic therapies (e.g., refractory response) may experience lower levels of efficacy with current biologic therapies compared with patients with no comorbidities or who are naive to advanced therapies (Citation3,Citation4). Recent guidelines (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the European League Against Rheumatism, and the American College of Rheumatology/National Psoriasis Foundation) endorse a specialized treatment approach for patients with PsA and other underlying comorbidities (Citation5–7). However, an unmet need remains for new treatment options that provide durable efficacy in a variety of patients, across heterogeneous patient demographics and clinical characteristics.
Risankizumab is a fully humanized immunoglobulin G1 monoclonal antibody that specifically inhibits interleukin-23 by binding to the p19 subunit (Citation8). KEEPsAKE 1 and KEEPsAKE 2 are ongoing phase 3 studies evaluating the efficacy and safety of risankizumab in patients with active PsA. The primary results at week 24 demonstrated efficacy based on ≥20% improvement in American College of Rheumatology criteria (ACR20) with risankizumab treatment (51.3%–57.3%) compared with placebo (26.5%–33.5%) in patients with active PsA (Citation9,Citation10). Additionally, continued risankizumab treatment demonstrated long-term efficacy (58.5%–70.0%) through 52 weeks based on the proportion of patients achieving ACR20 and improvements from weeks 24 to 52 in patients who switched from placebo to risankizumab (Citation11,Citation12). Herein, we evaluated the long-term efficacy (e.g., improvements in joint symptoms, skin resolution, disease activity, pain) of risankizumab across clinically relevant patient subgroups stratified by age, sex, body mass index, race, and disease characteristics through 52 weeks of treatment.
Methods
Study design
Detailed descriptions of KEEPsAKE 1 (NCT03675308) and KEEPsAKE 2 (NCT03671148) study designs and patient populations were previously reported (Citation9–12). Briefly, enrolled patients were aged ≥18 years; had a confirmed clinical diagnosis of PsA (symptom onset ≥6 months prior to screening, met the classification criteria for PsA and active disease [defined as ≥5 tender joints based on 68 joint counts with ≥5 swollen joints based on 66 joint counts, and active plaque or nail psoriasis]); had an inadequate response or intolerance to conventional synthetic disease-modifying, anti-rheumatic drugs (csDMARD; KEEPsAKE 1 and KEEPsAKE 2 studies); and/or had an inadequate response or intolerance to one or two biologic therapies (KEEPsAKE 2 study). Patients were randomized 1:1 to receive double-blind, subcutaneous risankizumab 150 mg or placebo at weeks 0, 4, and 16. At week 24, patients previously randomized to placebo received their first double-blinded dose of risankizumab 150 mg, and patients randomized to risankizumab received a single double-blinded dose of placebo. Starting on week 28, all patients were eligible to receive open-label risankizumab 150 mg every 12 weeks for up to 52 weeks.
The clinical trials were conducted in accordance with the protocol, International Council for Harmonization guidelines, and applicable guidelines and regulations governing ethical principles and study conduct originating in the Declaration of Helsinki. Independent ethics committees/institutional review boards ensured the ethical, scientific, and medical appropriateness of the study before it was conducted and approved all relevant documentation including the protocol, informed consent form(s), and all participant materials. Written informed consent was obtained from all patients before enrollment.
Assessments
Efficacy outcomes at weeks 24 and 52 were stratified and evaluated by baseline demographics (age [<65, ≥65 years], sex [male, female], body mass index [BMI; <25, ≥25 to <30, ≥30 kg/m2], race [White, non-white]), disease characteristics (duration since diagnosis of PsA [≤5, >5 to ≤10, >10 years], baseline high-sensitivity C-reactive protein levels [<3, ≥3 mg/L], swollen joint count [≤10, ≥10], tender joint count [≤20, ≥20]), and prior medication use (concomitant csDMARD use at baseline [any csDMARD, any methotrexate, none] and prior biologic use [yes, no]). Efficacy outcomes included joint symptoms improvement (≥20%/50%/70% improvement in ACR criteria [ACR20/50/70]; resolution of enthesitis and dactylitis [defined as patients with baseline Leeds enthesitis index score (LEI)≥0 or Leeds dactylitis index score (LDI)≥0, respectively, who achieved LEI = 0 and LDI = 0]) (Citation13, Citation14), skin clearance (≥90% improvement in Psoriasis Area and Severity Index [PASI90]) (Citation15), disease severity (minimal disease activity and Disease Activity in Psoriatic Arthritis Low Disease Activity [DAPSA LDA; DAPSA score of 5–14]) (Citation16, Citation17), and patient-reported outcomes (minimal clinically important difference in pain [≥10-mm decrease on a 100-mm visual analog scale]) (Citation18).
Statistical analysis
Efficacy outcomes at weeks 24 and 52 were analyzed by subgroups defined by baseline demographics, disease characteristics, and medication use (see Assessments section). Baseline demographic and clinical characteristics were evaluated using descriptive statistics (e.g., mean and standard deviation [SD], counts and percentages). For this efficacy analysis, 95% confidence intervals (CI) were calculated based on normal approximation to the binomial distribution and determined using the Cochran-Mantel-Haenszel test adjusting for the stratification factors of current use of csDMARD (0 vs. ≥1), presence of dactylitis (yes vs. no), presence of enthesitis (yes vs. no), and extent of psoriasis (≥3% or <3% body surface area) at baseline. For the week 24 analysis, missing data were imputed using non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; patients with missing data due to reasons other than COVID-19 or after intercurrent events (i.e., initiation of rescue medication or concomitant medications that could meaningfully impact efficacy assessment) were imputed as non-responders. Analysis performed at week 52 used non-responder imputation (as observed with imputation) that imputed data on patients with missing values as non-responders; intercurrent events were not considered for the long-term analysis.
Results
Patients
The full disposition of patients in the KEEPsAKE 1 and 2 studies have been previously reported (Citation11, Citation12). Briefly, among 1408 patients initially randomized to receive either risankizumab 150 mg or placebo, 1354 continued into the open-label periods; 1235 patients were still ongoing at time of data cutoff date (19 April 2021) and were included in this integrated analysis. Baseline demographics and clinical characteristics were generally well-balanced between groups (). In this integrated analysis, at baseline (range of values across treatment group and study), approximately half of the patients were female (47.8%−55.4%), the mean (SD) patient ages were 51.2 (12.1) to 53.1 (12.5) years, the mean (SD) BMI scores were 30.3 (6.2) to 31.5 (8.0) kg/m2, and the mean (SD) PASI scores were 7.7 (6.7) to 10.9 (10.1).
Table 1. Baseline demographics and clinical characteristics.
Efficacy
Week 24
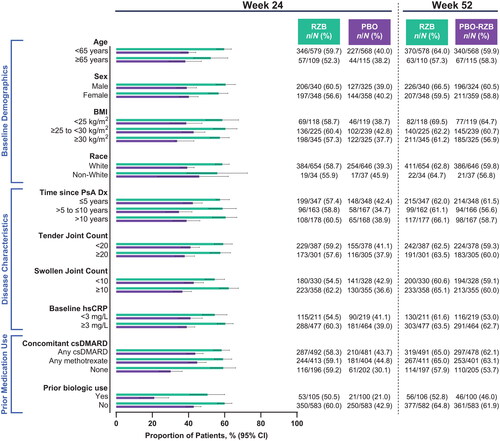
Across all subgroups, a numerically greater proportion of patients treated with risankizumab achieved ACR20 (the primary endpoint of the KEEPsAKE 1 and KEEPsAKE 2 studies) at week 24 compared with patients who received placebo (risankizumab, 46.3%−60.1%; placebo, 15.5%−36.2%; ). A generally consistent proportion of patients initially randomized to receive risankizumab achieved ACR20 at week 24, regardless of subgroup. Similar results were also observed for the achievement of secondary endpoints in the KEEPsAKE 1 and KEEPsAKE 2 studies of ACR50 (risankizumab, 19.8%−37.8%; placebo, 5.1%−13.7%; ) and ACR70 (risankizumab, 6.5%−18.0%; placebo, 2.2%−6.2%; ). Across all subgroups assessed, a greater proportion of patients treated with risankizumab experienced resolution of enthesitis (38.8%–60.8%) and dactylitis (57.4%–82.5%) compared with patients who received placebo (26.4%–41.2% and 37.3%–66.0%, respectively, Supplemental Figures 1–2).
Figure 1. Proportion of patients achieving ACR20 by subgroup. ACR20: ≥20% improvement in American College of Rheumatology criteria; BMI: body mass index; CI: confidence interval; csDMARD: conventional synthetic disease-modifying, anti-rheumatic drug; Dx: diagnosis; hsCRP: high-sensitivity C-reactive protein; PsA: psoriatic arthritis; PBO: placebo; RZB: risankizumab.
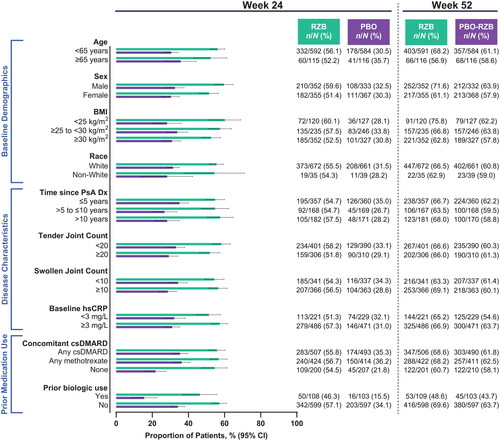
Figure 2. Proportion of patients achieving ACR50 by subgroup. ACR50: ≥50% improvement in American College of Rheumatology criteria; BMI: body mass index; CI: confidence interval; csDMARD: conventional synthetic disease-modifying, anti-rheumatic drug; Dx: diagnosis; hsCRP: high-sensitivity C-reactive protein; PsA: psoriatic arthritis; PBO: placebo; RZB: risankizumab.
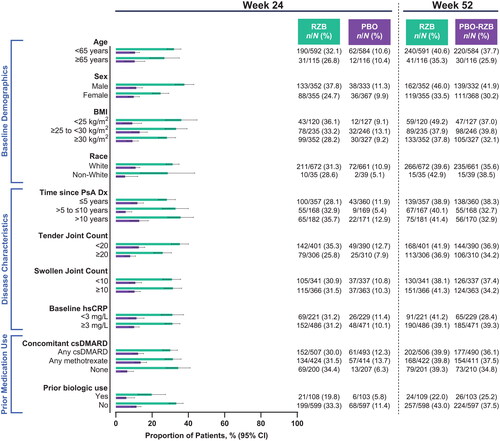
Figure 3. Proportion of patients achieving ACR70 by subgroup. ACR70: ≥70% improvement in American College of Rheumatology criteria; BMI: body mass index; CI: confidence interval; csDMARD: conventional synthetic disease-modifying, anti-rheumatic drug; Dx: diagnosis; hsCRP: high-sensitivity C-reactive protein; PsA: psoriatic arthritis; PBO: placebo; RZB: risankizumab.
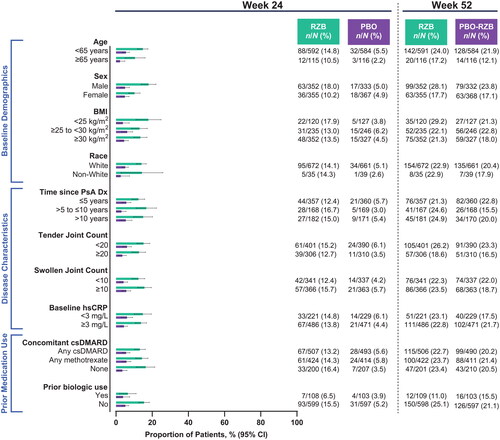
Among patients with ≥3% body surface area affected by psoriasis at baseline, numerically higher rates of achieving PASI90 with risankizumab vs. placebo at week 24 were observed across all subgroups (risankizumab, 44.4%−70.7%; placebo, 0%−16.1%; ). As was demonstrated for skin efficacy outcomes, the benefit of risankizumab in composite measures of disease activity over placebo was demonstrated, regardless of subgroups. At week 24, 15.0%−34.0% of patients receiving risankizumab and 4.5%−16.2% of patients receiving placebo achieved minimal disease activity (). DAPSA LDA was also achieved by a greater proportion of patients receiving risankizumab (14.5%−33.4%) compared with patients receiving placebo (5.9%−23.2%; ). Among patients with baseline pain, risankizumab-treated patients achieved a minimal clinically important difference in pain more often than did placebo-treated patients in all subgroups (risankizumab, 50.5%–62.2%; placebo, 21.0%–45.9%; ).
Figure 4. Proportion of patients achieving PASI90 by subgroup. Patients included in the PASI90 analysis had BSA ≥3% at baseline. BSA: body surface area; BMI: body mass index; CI: confidence interval; csDMARD: conventional synthetic disease-modifying, anti-rheumatic drug; Dx: diagnosis; hsCRP: high-sensitivity C-reactive protein; PASI90: ≥90% improvement in Psoriasis Area and Severity Index; PsA: psoriatic arthritis; PBO: placebo; RZB: risankizumab.
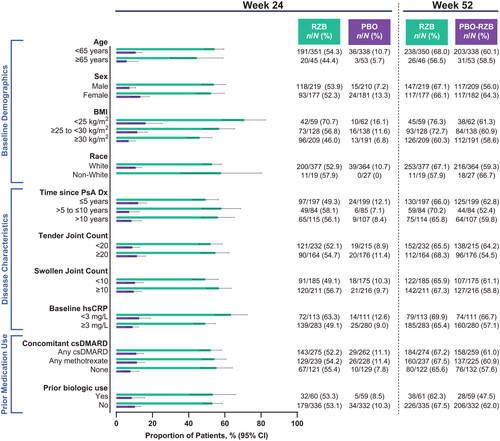
Figure 5. Proportion of patients achieving MDA by subgroup. BMI: body mass index; CI: confidence interval; csDMARD: conventional synthetic disease-modifying, anti-rheumatic drug; Dx: diagnosis; hsCRP: high-sensitivity C-reactive protein; MDA: minimal disease activity; PsA: psoriatic arthritis; PBO: placebo; RZB: risankizumab.
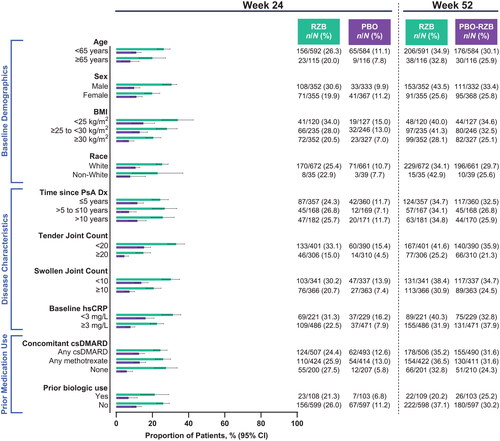
Figure 6. Proportion of patients achieving DAPSA LDA by subgroup. BMI: body mass index; CI: confidence interval; csDMARD: conventional synthetic disease-modifying, anti-rheumatic drug; DAPSA LDA: Disease Activity in Psoriatic Arthritis Low Disease Activity score of 5–14; Dx: diagnosis; hsCRP: high-sensitivity C-reactive protein; PsA: psoriatic arthritis; PBO: placebo; RZB: risankizumab.
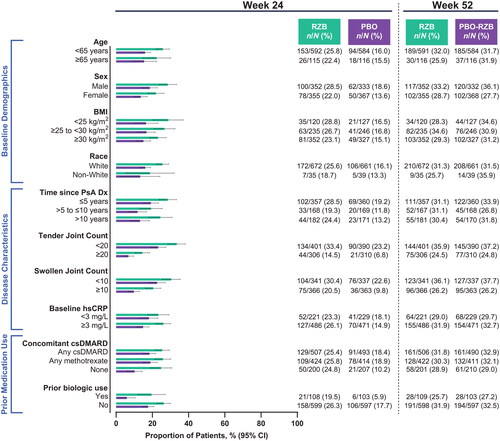
Figure 7. Proportion of patients achieving a minimal clinically important difference in pain by subgroup. A minimal clinically important difference in pain was defined as ≥10 mm decrease (on a 100-mm visual analog scale) among patients with baseline pain ≥10 mm. BMI: body mass index; csDMARD: conventional synthetic disease-modifying, anti-rheumatic drug; CI: confidence interval; Dx: diagnosis; hsCRP: high-sensitivity C-reactive protein; PsA: psoriatic arthritis; PBO: placebo; RZB: risankizumab.
Week 52
Response rates in all efficacy assessments at week 52 were generally maintained (in patients who were initially randomized to risankizumab) or increased (in those who received placebo through week 24) across all subgroups and efficacy outcomes (). Patients treated with risankizumab throughout the study achieved generally consistent efficacy, regardless of patient demographic, disease characteristics, and prior/concomitant treatment exposure. At week 52, patients who switched from placebo to risankizumab also achieved similar response rates as those who were treated with risankizumab from baseline through week 52 with similar improved efficacy across subgroups.
Discussion
These integrated analyses from the KEEPsAKE 1 and KEEPsAKE 2 studies demonstrate that efficacy was consistently achieved with risankizumab treatment through 52 weeks across subgroups stratified by demographics, disease characteristics, and prior/concomitant treatments, and were similar to levels of efficacy observed in the general study population (Citation9–12). Among all subgroups, numerically higher response rates were achieved in patients randomized to receive risankizumab relative to those patients who received placebo at week 24 across disease domains, including rigorous skin response criteria, composite measures to assess overall disease activity, and PsA-related signs and symptoms. Furthermore, patients randomized to receive continued risankizumab treatment achieved sustained response rates through 52 weeks. As expected, patients who were randomized to placebo at baseline experienced efficacy improvements from when they switched to risankizumab (at week 24) to week 52 across all subgroups. The lack of an apparent subgroup effect on efficacy outcomes suggests that patients with varying baseline demographics and clinical characteristics may experience improvement of joint symptoms with risankizumab treatment at weeks 24 and 52. Given the varied clinical manifestations and heterogeneity of PsA, measuring disease activity can be difficult (Citation19). A numerically greater proportion of risankizumab-treated patients achieved both MDA and DAPSA LDA from week 24 to 52 vs. those who received placebo, regardless of subgroups, which may further highlight that patients with a range of disease characteristics may continue to achieve low levels of disease activity with risankizumab.
Despite the availability of treatment options, PsA remains undertreated, especially in patients with underlying comorbidities. Several well-known predisposing factors can affect PsA treatment response (Citation3,Citation4). In a study evaluating a tumor necrosis factor inhibitor, researchers found that the likelihood of achieving an efficacious response was generally lower in patients who are overweight or obese than among patients with normal body weight (Citation20). Another well-known factor that could negatively affect efficacy is prior exposure to biologic therapy (Citation21). Patients who have a history of biologic use are generally more often refractory to treatment compared with patients who were naive to biologic treatment. Not surprisingly, in these studies, higher BMI (≥30 kg/m2) and prior exposure to biologic therapy were associated with numerically lower response rates across all efficacy outcomes with risankizumab treatment, though no inferential statistical testing was performed to compare different subgroups. In this post hoc analysis, however, more patients treated with risankizumab compared with those treated with placebo showed improvements in PsA signs and symptoms at weeks 24 and 52, regardless of BMI and prior biologic use.
Although our post hoc analysis lacked multiplicity control to conduct inferential statistical comparisons between subgroups receiving the same treatments, we did observe some nominal trends among patients in the following subgroups at week 24: sex, BMI, swollen joint count, tender joint count, and prior biologic use. These differences were numeric only. Analyses may be conducted in the future to evaluate and confirm whether numerical differences are meaningful between subgroups of patients treated with risankizumab.
Although pooled safety data were not evaluted in this post hoc analysis, long-term safety data from the KEEPsAKE 1 and KEEPsAKE 2 studies has been published showing that risankizumab was generally well tolerated through 52 weeks (Citation11,Citation12).
This post hoc analysis is not without limitations. The relatively small sample sizes in a few subgroups (e.g., non-white race, aged ≥65 years, prior biologic use) may have led to a lack of precision in estimating treatment effects. Secondly, while key subgroups were selected to reflect real-world populations, this analysis remains a post hoc assessment of clinical trial populations; further evidence based on real-world patient data may be needed to confirm if the overall results are translatable to the general population.
In conclusion, risankizumab consistently resulted in greater efficacy compared with placebo at week 24 and sustained or improved efficacy at week 52 across different subgroups, regardless of baseline demographics and disease characteristics, or concomitant and prior medication use. These efficacy results support long-term use of risankizumab for patients with active PsA across multiple patient and psoriatic disease characteristics.
Contributions
J. F. Merola, A. Armstrong, S. Khattri, S. Y. Paek, B. Padilla, C. Yue, H. Photowala, B. Kaplan, and L. E. Kristensen contributed to data interpretation, critically reviewed this manuscript, and provided final approval for publication. B. Padilla, C. Yue, H. Photowala, and B. Kaplan contributed to the study concept and design. C. Yue participated in statistical analysis. L. E. Kristensen participated in data acquisition.
Ethics statement
The Independent Ethics Committee or Institutional Review Board at each study site approved the study protocol, informed consent forms, and recruitment materials before patient enrollment. The studies were conducted in accordance with the International Conference for Harmonization guidelines, applicable regulations, and the Declaration of Helsinki. All patients provided written informed consent before screening.
Supplemental Material
Download PDF (141.6 KB)Acknowledgments
AbbVie and authors thank all the trial investigators and the patients who participated in this clinical trial.
Disclosure statement
J. F. Merola reports consultant or investigator work for AbbVie, Amgen, Biogen, BMS, Dermavant, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB.
A. Armstrong has received research grants from AbbVie, BMS, Dermira, Janssen, Kyowa Hakko Kirin, Lilly, Novartis, and UCB; and has received personal fees from AbbVie, BI/Parexel, BMS, Celgene, Dermavant, Genentech, GSK, Janssen, Lilly, Menlo Therapeutics, Merck, Modernizing Medicine, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Science 37, Sun Pharma, and Valeant.
S. Khattri is a speaker for AbbVie, Janssen, Lilly, and UCB; serves as an advisory board member/consultant for AbbVie, Janssen, Lilly, Novartis, and UCB; and has received research grants from AbbVie, BMS, LEO, Novartis, and Pfizer.
S. Y. Paek has received fees for serving as a speaker or consultant from AbbVie, BMS, Janssen, Novartis, Sanofi Genzyme, and UCB. She has received research grants from AbbVie, Amgen, and Incyte.
B. Padilla, C. Yue, H. Photowala, and B. Kaplan are employees of AbbVie, and may hold AbbVie stock and/or stock options.
L. E. Kristensen has received honoraria or fees for serving as a speaker or consultant from AbbVie, Amgen, Biogen, BMS, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB. He has received investigator-initiated study grants from AbbVie, Biogen, Janssen, Lilly, Novartis, Pfizer, and UCB.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. These data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select ‘Home’.
Additional information
Funding
References
- FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7(1):1. doi:10.1038/s41572-021-00293-y.
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545–11. doi:10.1016/j.rdc.2015.07.001.
- Kumthekar A, Ogdie A. Obesity and psoriatic arthritis: a narrative review. Rheumatol Ther. 2020;7(3):447–−456. doi:10.1007/s40744-020-00215-6.
- Mease PJ, Karki C, Liu M, et al. Baseline patient characteristics associated with response to biologic therapy in patients with psoriatic arthritis enrolled in the corrona psoriatic arthritis/spondyloarthritis registry. RMD Open. 2018;4(1):e000638. doi:10.1136/rmdopen-2017-000638.
- Coates LC, Soriano ER, Corp N, et al. Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–479. doi:10.1038/s41584-022-00798-0.
- Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700.1–712. doi:10.1136/annrheumdis-2020-217163.
- Singh JA, Guyatt G, Ogdie A, et al. 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken). 2019;71(1):2–29. doi:10.1002/acr.23789.
- Skyrizi (risankizumab-rzaa) prescribing information. North Chicago (IL): AbbVie Inc.; 2024. [accessed 2024 April 10]. Available from https://www.rxabbvie.com/pdf/skyrizi_pi.pdf
- Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81(2):225–231. doi:10.1136/annrheumdis-2021-221019.
- Östör A, Van den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis. 2022;81(3):351–358. doi:10.1136/annrheumdis-2021-221048.
- Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 1 study. Rheumatology (Oxford). 2022;62(6):2113–2121. doi:10.1093/rheumatology/keac607.
- Östör A, Van den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 2 study. Rheumatology (Oxford). 2022;62(6):2122–2129. doi:10.1093/rheumatology/keac605.
- Felson DT, Anderson JJ, Boers M, et al. American college of rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–735. doi:10.1002/art.1780380602.
- Felson DT, Anderson JJ, Lange ML, et al. Should improvement in rheumatoid arthritis clinical trials be defined as fifty percent or seventy percent improvement in core set measures, rather than twenty percent? Arthritis Rheum. 1998;41(9):1564–1570. doi:10.1002/1529-0131(199809)41:9%3C1564::AID-ART6%3E3.0.CO;2-M.
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. Acad Dermatol Venereol. 2015;29(4):645–648. doi:10.1111/jdv.12817.
- Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(01):48–53. doi:10.1136/ard.2008.102053.
- Schoels MM, Aletaha D, Alasti F, et al. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–818. doi:10.1136/annrheumdis-2015-207507.
- Strand V, Boers M, Idzerda L, et al. It’s good to feel better but it’s better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol. 2011;38(8):1720–1727. doi:10.3899/jrheum.110392.
- Leung YY, Ogdie A, Orbai AM, et al. Classification and outcome measures for psoriatic arthritis. Front Med. 2018;5:246. doi:10.3389/fmed.2018.00246.
- Eder L, Thavaneswaran A, Chandran V, et al. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis. 2015;74(5):813–817. doi:10.1136/annrheumdis-2013-204448.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi:10.1016/j.jaad.2018.11.057.

