Abstract
Purpose: Atopic dermatitis (AD) adversely impacts quality of life (QoL). We evaluated the effect of upadacitinib, an oral selective Janus kinase inhibitor approved for moderate-to-severe AD, plus topical corticosteroids (+TCS) on patient-reported outcomes (PROs) over 52 weeks.
Materials and methods: In the phase 3 AD Up study (NCT03568318), adults and adolescents with moderate-to-severe AD were randomized 1:1:1 to once-daily upadacitinib 15 mg, 30 mg, or placebo + TCS. Itch, skin pain/symptoms, sleep, QoL, daily activities, emotional state, mental health, and patient impressions of disease severity/improvement/treatment satisfaction were assessed.
Results: This analysis included 901 patients. Within 1–2 weeks, PRO improvements were greater with both upadacitinib doses than with placebo (p <.05). Improvements increased through weeks 4–8; rates were generally maintained through week 52. At week 52, the proportion of patients with clinically meaningful improvements in itch (Worst Pruritus Numerical Rating Scale improvement ≥4), skin pain (AD Symptom Scale Skin Pain improvement ≥4), sleep (AD Impact Scale [ADerm-IS] Sleep improvement ≥12), daily activities (ADerm-IS Daily Activities improvement ≥14), and emotional state (ADerm-IS Emotional State improvement ≥11) ranged from 62.1%–77.7% with upadacitinib 15 mg + TCS and 71.3%–83.6% with upadacitinib 30 mg + TCS.
Conclusions: Upadacitinib + TCS results in rapid, sustained improvements in burdensome AD symptoms and QoL.
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin condition characterized by intense itch and reoccurring eczematous lesions (Citation1). AD effects up to 20% of children and 10% of adults, with an estimated global prevalence rate of 2960 per 100,000 people (Citation2). AD symptoms negatively affect patients’ health-related quality of life (HRQoL), leading to sleep disturbance, social avoidance, worsened mental health, and disruption of daily activities; more severe AD symptoms correlate with worse HRQoL outcomes (Citation3–5).
Patient-reported outcomes (PROs) provide important insights into the multidimensional burden of AD, including itch, pain, and quality of life (QoL) (Citation6,Citation7). Information obtained from PROs complements information obtained from validated clinician-assessed outcome measures, providing a comprehensive understanding of patients’ experience (Citation6). Using PROs in both clinical trials and real-world care for AD facilitates patient input and shared decision-making and allows the symptom burden and subsequent treatment impact on HRQoL to be fully recognized (Citation6,Citation7). PROs are emphasized as a critical core outcome for AD clinical trials by the international Harmonizing Outcome Measures for Eczema (HOME) consensus group, which recommends the assessment of itch and other skin symptoms, QoL, and patient impressions of control of AD (Citation8). PROs are also recommended to guide treatment decisions to provide a more holistic approach in AD treat-to-target guidelines (Citation9,Citation10), HOME clinical practice guidelines (Citation11,Citation12), and the recently developed minimal disease activity criteria (Citation13).
Upadacitinib is an oral selective Janus kinase (JAK) inhibitor that has greater inhibitory potency for JAK1 than JAK2, JAK3, and tyrosine kinase 2 (Citation14,Citation15). Upadacitinib demonstrated efficacy and safety in patients with moderate-to-severe AD both alone and in combination with topical corticosteroids (TCS) (Citation16–20). Dysregulated JAK signaling is implicated in AD; JAKs mediate signaling of many cytokines involved in AD, including interleukin (IL)-4, IL-13, IL-31, and thymic stromal lymphopoietin, which may contribute to hallmark symptoms including itch (Citation21,Citation22). Upadacitinib is approved for the treatment of moderate-to-severe AD in adults and adolescents (Citation14,Citation15). Using data from AD Up (NCT03568318), a phase 3, randomized, placebo-controlled controlled clinical trial (Citation19,Citation20), we evaluated the effects of upadacitinib plus TCS on PROs in adults and adolescents with moderate-to-severe AD.
Materials and methods
Study design and patients
Data from the AD Up study were used for this analysis. A detailed description of the AD Up study design, patients, and methods were previously published (Citation19,Citation20). Briefly, AD Up was a randomized, double-blind, placebo-controlled, phase 3 clinical trial evaluating the efficacy and safety of upadacitinib in patients with moderate-to-severe AD who were receiving TCS. The AD Up clinical trial consisted of a 16-week, double-blind, placebo-controlled period followed by an ongoing blinded extension period for up to 260 weeks. The AD Up study was conducted at 171 clinical centers across 22 countries in Asia-Pacific, Europe, the Middle East, North America, and Oceania.
Eligible patients were aged 12–75 years with chronic, moderate-to-severe AD (≥10% of body surface area affected, Eczema Area and Severity Index [EASI] ≥ 16, Validated Investigator’s Global Assessment for AD [vIGA-AD] ≥ 3, and baseline weekly average Worst Pruritus Numerical Rating Scale [WP-NRS] score ≥4). Patients were randomized 1:1:1 to receive once-daily orally administered upadacitinib 15 mg, upadacitinib 30 mg, or placebo plus TCS. Randomization was stratified by AD severity (baseline vIGA-AD score 3 or 4), geographic region (US/Puerto Rico/Canada, Japan, China, or other), and age group (adult or adolescent [aged 12–17 years]). After week 16, patients who received a placebo were rerandomized 1:1 to receive upadacitinib 15 mg or upadacitinib 30 mg plus TCS in the ongoing blinded extension period. Twice-daily use of an additive-free bland emollient ≥7 d before baseline and through week 52 was required. Investigators, patients, and study site staff were blinded to treatments for the study duration.
Independent ethics committees or institutional review boards at each study site approved the study protocols, informed consent forms, and recruitment materials before patient enrollment. The studies were conducted in accordance with the International Conference for Harmonization guidelines, applicable regulations, and the Declaration of Helsinki. All patients provided written informed consent.
Assessments
The symptom and HRQoL disease burden, including itch, skin pain, other skin symptoms, sleep, QoL, and mental health, were measured using validated PRO assessment tools (Supplemental Table S1) (Citation23–28). Overall patient perspectives on symptoms and treatment were evaluated with the Patient Global Impression of Severity, Patient Global Impression of Change, and Patient Global Impression of Treatment tools (Citation29). PROs were collected electronically via questionnaires administered at home or select visits and were reported through week 52 of the blinded extension period (Supplemental Table S2). PROs were presented in two ways: (1) the proportion of patients achieving a clinically meaningful improvement, defined as the minimal clinically important difference from baseline (as previously applied in the literature (Citation26)) (Supplemental Table S1), and (2) the proportion of patients achieving a stringent PRO score reflective of minimal disease burden (representing no/minimal symptoms or no/minimal impact of AD on HRQoL) (Supplemental Table S1). PRO endpoints were prespecified, except for minimal disease burden threshold scores involving the Patient-Oriented Eczema Measure (POEM), the AD Impact Scale (ADerm-IS), and the AD Symptom Scale (ADerm-SS). PRO assessments were reported through week 16 for all treatment groups and through week 52 for patients who were initially randomized to upadacitinib plus TCS and continued upadacitinib plus TCS in the blinded extension period.
Statistical analysis
Outcomes were analyzed in the intent-to-treat population for the main study, defined as all patients who were randomized into the main study; this population is the same as that included in the primary publications of the AD Up study (Citation19,Citation20). All categorical outcomes through the 16-week double-blind period were evaluated using the Cochran-Mantel-Haenszel test. Data through week 16 were reported using nonresponder imputation incorporating multiple imputation for missing data due to COVID-19 (NRI-C): a patient with missing data at a visit was categorized as a nonresponder, with 2 exceptions: (1) if the patient was a responder before and after the timepoint with missing data, they were categorized as a responder, and (2) missing data due to COVID-19 were handled by multiple imputation. Long-term outcomes from week 0 through week 52 were descriptively reported using observed cases (OC) while patients were receiving treatment (i.e., no imputation of missing data was applied).
Results
Patients
Between August 9, 2018, and December 20, 2019, a total of 901 patients enrolled in the AD Up study and were included in this analysis. The baseline demographics and disease characteristics, including PROs, were generally balanced across the treatment groups ().
Table 1. Baseline demographics and characteristics for patients enrolled in the AD Up study.
PRO measures
Itch
By week 1, a significantly greater proportion of patients who received upadacitinib 15 mg or upadacitinib 30 mg plus TCS experienced rapid, clinically meaningful improvements in itch compared with patients who received placebo plus TCS (p <.001 vs placebo; NRI-C) (Supplemental Table S3). Response rates rapidly increased for patients who received either dose of upadacitinib plus TCS; by week 16, more than 50% of patients achieved a clinically meaningful improvement in itch with either dose of upadacitinib plus TCS, representing a significantly greater proportion compared with 15% of patients who received placebo plus TCS (p <.001 vs placebo; NRI-C). When evaluating long-term outcomes up to week 52 using OC, improvements in itch were generally maintained through week 52 ().
Figure 1. Improvement in itch through 52 weeks of treatment with upadacitinib plus topical corticosteroids. Error bars indicate 95% confidence interval. Data are represented as observed cases. aAssessed in patients with WP-NRS ≥4 at baseline. bAssessed in patients with WP-NRS ≥2 at baseline. PBO: placebo; TCS: topical corticosteroids; UPA: upadacitinib; WP-NRS: Worst Pruritus Numerical Rating Scale.
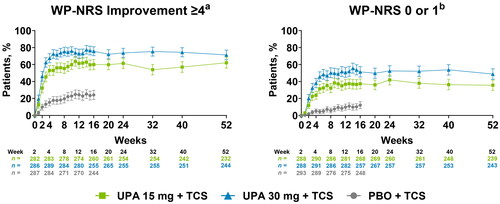
Similarly, rapid and sustained achievement of the stringent endpoint of no/minimal itch were reported in patients receiving upadacitinib 15 mg or upadacitinib 30 mg plus TCS (NRI-C) (Supplemental Table S3). By week 1, a greater proportion of patients reported no/minimal itch with either dose of upadacitinib plus TCS compared with patients receiving placebo plus TCS. By week 16, more than 30% and 40% of patients receiving upadacitinib 15 mg or upadacitinib 30 mg plus TCS, respectively, experienced no/minimal itch, compared with <10% of patients receiving placebo plus TCS. The proportion of patients achieving no/minimal itch was generally sustained through week 52 (OC) ().
Skin pain and other skin symptoms
By week 1, more patients receiving either dose of upadacitinib plus TCS experienced clinically meaningful improvements in skin pain and skin symptoms than patients receiving placebo plus TCS (NRI-C) (Supplemental Table S3). At week 16, more than 55% of patients receiving either dose of upadacitinib plus TCS experienced clinically meaningful improvements in skin pain and skin symptoms compared with ≤20% of patients receiving placebo plus TCS. Responses were similar for the proportion of patients achieving clinical meaningful improvements on the POEM. Improvements in skin pain, other skin symptoms, and disease severity were maintained through week 52 (OC) ().
Figure 2. Improvement in skin symptoms including pain through 52 weeks of treatment with upadacitinib plus topical corticosteroids. Error bars indicate 95% confidence interval. Data are represented as observed cases. aAssessed in patients with ADerm-SS Skin Pain ≥4 at baseline. bAssessed in patients with ADerm-SS TSS-7 ≥ 28 at baseline. cAssessed in patients with POEM ≥4 at baseline. dAssessed in patients with ADerm-SS Skin Pain ≥2 at baseline. eAssessed in patients with ADerm-SS TSS-7 ≥ 12 at baseline. fAssessed in patients with POEM ≥3 at baseline. ADerm-SS: Atopic Dermatitis Symptom Scale; PBO: placebo; POEM: Patient-Oriented Eczema Measure; TCS: topical corticosteroids; TSS-7: 7-item Total Symptom Score; UPA: upadacitinib.
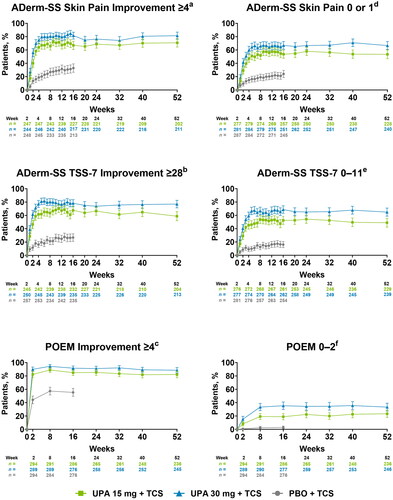
Similarly, stringent endpoints representing minimal disease burden for skin pain and other skin symptoms were rapidly achieved with both doses of upadacitinib plus TCS. More patients who received either dose of upadacitinib plus TCS vs placebo plus TCS reported no/minimal skin pain or skin symptoms by week 1. More patients who received either dose of upadacitinib plus TCS vs placebo plus TCS reported clear or almost clear disease on the POEM by week 2 (NRI-C) (Supplemental Table S3). Response rates continued to improve; at week 16, more than 45% of patients who received either dose of upadacitinib plus TCS reported no/minimal skin pain and skin symptoms, and more than 15% of patients reported clear or almost clear disease compared with <20% of patients who received placebo plus TCS. Rates of achievement of the stringent skin-related PROs were sustained through week 52 (OC) ().
Sleep
At week 1, a greater proportion of patients receiving either dose of upadacitinib plus TCS achieved clinically meaningful improvements in sleep compared with patients who received placebo plus TCS (NRI-C) (Supplemental Table S3). By week 16, more than 60% of patients who received either dose of upadacitinib plus TCS achieved clinically meaningful improvements in sleep compared with <25% of patients receiving placebo plus TCS. Rates of sleep improvement were maintained through week 52 (OC) ().
Figure 3. Improvement in sleep through 52 weeks of treatment with upadacitinib plus topical corticosteroids. Error bars indicate 95% confidence interval. Data are represented as observed cases. aAssessed in patients with ADerm-IS Sleep score ≥12 at baseline. bAssessed in patients with ADerm-IS Sleep score ≥4 at baseline. ADerm-IS: Atopic Dermatitis Impact Scale; PBO: placebo; TCS: topical corticosteroids; UPA: upadacitinib.
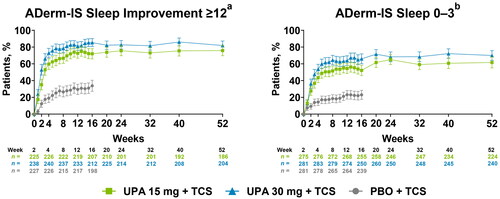
Achievement of the stringent endpoints of no/minimal sleep disturbance were rapid and sustained with upadacitinib plus TCS vs placebo plus TCS, with greater response rates observed for ADerm-IS Sleep score of 0–3 at week 1 and POEM Sleep score of 0 at week 2 with either dose of upadacitinib plus TCS (NRI-C) (Supplemental Table S3). By week 16, more than 40% of patients who received either dose of upadacitinib plus TCS reported no/minimal sleep disturbance compared with <15% of patients who received placebo plus TCS; response rates were sustained through week 52 (OC) ( and Supplemental Figure S1).
Quality of life and mental health
A greater proportion of patients who received either dose of upadacitinib plus TCS vs placebo plus TCS experienced clinically meaningful improvements in daily activities and emotional state by week 1 and QoL by week 2 (NRI-C) (Supplemental Table S3). At week 16, more than 80% of patients experienced clinically meaningful improvements in QoL, and more than 60% of patients experienced clinically meaningful improvements in daily activities and emotional state with either dose of upadacitinib plus TCS compared with <45% of patients who received placebo plus TCS. Rates of clinically meaningful improvement in QoL were maintained through week 52 (OC) ().
Figure 4. Improvements in quality of life, daily activities, and emotional state through 52 weeks of treatment with upadacitinib plus topical corticosteroids. Error bars indicate 95% confidence interval. Data are represented as observed cases. aAssessed in patients with DLQI ≥4 at baseline. bAssessed in patients with ADerm-IS Daily Activities score ≥14 at baseline. cAssessed in patients with ADerm-IS Emotional State score ≥11 at baseline. dAssessed in patients with DLQI ≥2 at baseline. eAssessed in patients with ADerm-IS Daily Activities score ≥3 at baseline. fAssessed in patients with ADerm-IS Emotional State score ≥3 at baseline. ADerm-IS: Atopic Dermatitis Impact Scale; DLQI: Dermatology Life Quality Index; PBO: placebo; TCS: topical corticosteroids: UPA: upadacitinib.
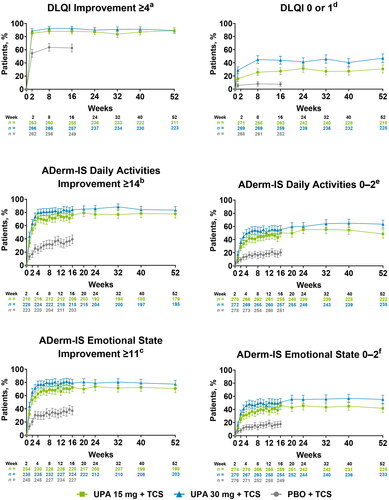
At week 2, a greater proportion of patients who received either dose of upadacitinib plus TCS reported no/minimal impact of AD on QoL, daily activities, and emotional state compared with patients who received placebo plus TCS (NRI-C) (Supplemental Table S3). By week 16, more than 25% of patients reported no impact on QoL, and more than 40% of patients reported no/minimal impact on daily activities and emotional state with either dose of upadacitinib plus TCS compared with <15% for patients receiving placebo plus TCS. Rates of achieving the stringent endpoints of no/minimal impact on QoL, daily activities, and emotional state were generally sustained through week 52 (OC) ().
Nearly half of the patients who received either dose of upadacitinib plus TCS experienced clinically meaningful improvements on the Hospital Anxiety and Depression Scale by week 16 compared with <30% of patients who received placebo plus TCS (NRI-C) (Supplemental Table S3). Improvements in anxiety and depression with either dose of upadacitinib plus TCS were maintained through week 52 (OC) (Supplemental Figure S2).
Patient impressions of disease severity and treatment
Patients in both upadacitinib plus TCS groups reported greater improvements from baseline in impressions of disease severity, disease improvements, and treatment satisfaction than patients in the placebo plus TCS group by week 2 (NRI-C) (Supplemental Table S3). At week 16, more than 40% of patients experienced absent or minimal symptoms on the Patient Global Impression of Severity scale, more than 70% reported ‘very much’ or ‘much improved’ change in symptoms on the Patient Global Impression of Change scale, and more than 50% reported they were ‘extremely satisfied’ or ‘very satisfied’ with treatment on the Patient Global Impression of Treatment scale with either dose of upadacitinib plus TCS compared with <30% of patients receiving placebo plus TCS. Rates were sustained through week 52 (OC) (Supplemental Figure S3).
Discussion
Clinical trials evaluating treatments for patients with AD often rely heavily on clinician-reported assessments of skin clearance such as EASI and vIGA-AD. However, clinicians’ assessments of skin symptoms often provide a limited snapshot of the multidimensional burden experienced by patients with AD (Citation6,Citation30). Thus, PROs assessing itch, skin pain, sleep, QoL, daily activities, emotional state, and mental health become critical to consider, complementing the information obtained from objective clinical assessments (Citation6).
Adults and adolescents with moderate-to-severe AD who received once-daily orally administered upadacitinib 15 mg or upadacitinib 30 mg plus TCS demonstrated rapid improvements and sustained response rates in PRO measures including itch, skin pain and other skin symptoms, sleep, mental health, and QoL. At the earliest weekly timepoints assessed, patients who received either dose of upadacitinib plus TCS generally experienced greater improvements in PROs when compared with patients who received placebo plus TCS. The proportion of patients experiencing improvements in PROs with either dose of upadacitinib plus TCS rapidly increased following treatment initiation and generally plateaued around weeks 4–8, with response rates sustained through week 52. Patients in the upadacitinib plus TCS groups generally reported rapid improvements and sustained response rates across PROs when measured either by the minimal clinically important differences or the more stringent threshold scores representing minimal disease burden; patient impressions of disease severity and treatment followed a similar trend. Generally, rates of improvement in PROs were numerically higher for patients who received upadacitinib 30 mg plus TCS compared with patients who received upadacitinib 15 mg plus TCS.
This study evaluates the achievement of minimal disease burden thresholds for PROs representing no or minimal impact of AD on patients’ lives, reflecting more stringent endpoints than the achievement of minimal clinically important differences. Despite an increased appreciation for the utility of PROs in assessing treatment impact in both routine care and clinical trials (Citation8–10,Citation13), there is no clear consensus on how to evaluate improvements in PROs or how to effectively utilize PROs to guide treatment decisions (Citation11,Citation12). When applying the stringent minimal disease burden threshold scores in this study, upadacitinib plus TCS improved PRO achievement rates compared with placebo plus TCS. Incorporating highly stringent treatment goals for PROs, such as those presented herein, may help reduce the burden of AD on patients and improve QoL.
In the AD Up study, treatment with upadacitinib plus TCS resulted in rapid improvements in both PROs and key clinical outcomes (≥75% improvement from baseline in EASI and vIGA-AD 0 or 1) (Citation19,Citation20). The temporal course of improvements in PROs and clinical outcomes were similar, with rapid improvements continuing through weeks 4–8, and rates generally sustained through week 52 (Citation19,Citation20). Additionally, an integrated post hoc analysis of upadacitinib in patients with moderate-to-severe AD from three clinical trials, including the AD Up study, reported that higher levels of clinician-assessed skin clearance correlated with greater improvements in PROs encompassing itch and other skin symptoms, sleep, QoL, and mental health (Citation26). The concurrent improvements in PROs and clinical measures in AD Up, and the correlation between improvements in PROs and clinical measures previously reported (Citation26), underscore the value of using PROs to assess the treatment impact of AD therapies. While there is a correlation between improvements in PROs and clinical measures among patients with AD, assessing PROs also provides a more nuanced understanding of the disease burden for individual patients; for example, not all patients who achieved high levels of clinician-assessed skin clearance with upadacitinib also achieved clinically meaningful improvements in scores on the Hospital Anxiety and Depression Scale and Dermatology Life Quality Index (Citation26). Furthermore, utilizing PROs when assessing treatment impact aligns with recent AD clinical trial guidelines (Citation8), treat-to-target and clinical practice guidelines (Citation9,Citation10,Citation12), and the minimal disease activity criteria (Citation13).
In the AD Up study, upadacitinib plus TCS rapidly controlled itch in patients with moderate-to-severe AD, with improvements seen by week 1 (the earliest timepoint reported) (Citation20). Upadacitinib monotherapy similarly resulted in rapid control of itch in the Measure Up 1 and Measure Up 2 phase 3 studies, with improvements occurring within 1–2 d (Citation17). Itch is a critical symptom to assess as it underlies many of the negative effects of AD on QoL, including sleep disturbance (Citation31,Citation32). In accordance, the rapid improvements in itch observed with upadacitinib plus TCS were concurrent with rapid improvements in sleep. Sleep disturbances are associated with worsened QoL, increased anxiety and depression, and compromised ability to work (Citation31–33). Thus, improving itch and sleep in patients with AD may lead to broader improvements in overall QoL and well-being. The rapid and concurrent improvements in itch and sleep with upadacitinib plus TCS are important factors to consider for patients with AD when making treatment decisions.
Previous reports of the AD Up study describe upadacitinib plus TCS as having a favorable safety profile through week 52 (Citation19). Adverse events and serious adverse events occurred at similar rates in the upadacitinib 15 mg and upadacitinib 30 mg plus TCS groups. No new safety signals were identified.
Limitations of this analysis include the interdependence of PROs (e.g., itch, sleep, and QoL), which make it challenging to differentiate improvements within distinct PRO categories. Additionally, long-term outcomes past week 16 do not have a placebo comparator. Thus, the descriptive data from the blinded extension period past week 16 should be interpreted carefully as they lack a control group. The stringent NRI-C approach, which applies a nonresponse when data are missing or unavailable, was only applied through week 16 (Supplemental Table S3). The Cochran-Mantel-Haenszel test was used to measure statistical differences between treatment groups through week 16. Long-term outcomes through week 52 were reported descriptively as OC, with no imputation for missing data; therefore, this may lead to overestimating the effects of upadacitinib treatment. Due to the lack of a control group and the OC imputation approach, long-term outcomes from week 16 through week 52 should be interpreted more cautiously.
When treating patients with AD, it is important to incorporate both objective clinical outcomes and PROs into shared decision-making conversations with patients, as this may increase treatment satisfaction, avoid undertreatment, and optimize care (Citation6,Citation7,Citation9,Citation13). Our findings demonstrate that in patients with moderate-to-severe AD receiving TCS, upadacitinib rapidly reduces the patient-reported disease burden and improves HRQoL, with sustained effects through 52 weeks of treatment.
Authors contributions
All authors have critically reviewed the manuscript and approved the final version for submission. BMC, HT, and XH participated in study concept/design. NM, HT, XH, and RGL participated in data acquisition. XH, YY, YL, and SZ participated in statistical analysis. NM, MCC, MS, BG, BMC, HT, CSS, KA, and RGL participated in data interpretation. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (134.6 KB)Acknowledgments
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. AbbVie and authors thank all the trial investigators and the patients who participated in this clinical trial.
Medical writing support was provided by Morgan A. Gingerich, PhD, and Akua Adu-Boahene, MD, MPH, of JB Ashtin, and funded by AbbVie.
Disclosure statement
N Magnolo has received honoraria as an advisor, speaker, and/or consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Janssen, La Roche-Posay, LEO Pharma, Lilly, Novartis, Pfizer, Dr. Wolff, and UCB Pharma. MC Cameron has received honoraria as a consultant or speaker for AbbVie, Amgen, Bristol Myers Squibb, Dermavant, Evelo, Incyte, Journey Medical, LEO Pharma, Lilly, Ortho Pharmaceutical, Pfizer, Regeneron, and Verrica. M Shahriari has received honoraria as a consultant for AbbVie, Amgen, Arcutis, BMS, Dermavant, Galderma, Incyte, Janssen, LEO Pharma, Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi-Genzyme; has served as a speaker for AbbVie, Arcutis, BMS, Dermavant, Lilly, Janssen, Pfizer, Sanofi-Genzyme, Regeneron, and UCB; and has served as an investigator for AbbVie, Cara, the Corrona Atopic Dermatitis Registry, Dermavant, Dermira, and Novartis. B Geng has served as an advisor, consultant, speaker and/or researcher for ADMA Biologics Inc., AstraZeneca, BioCryst, Chiesi, Galderma, GlaxoSmithKline, Grifols, Horizon, Koru, Novartis, OptiNose, Pfizer, Regeneron, Sanofi, Takeda, and Teva. BM Calimlim, H Teixeira, X Hu, Y Yang, Y Liu, S Zhang, C Sancho Sanchez, and K Altman are full-time employees of AbbVie and may hold AbbVie stock or stock options. RG Langley has received honoraria as an investigator, speaker, and/or consultant for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, Merck, Novartis, Pfizer, and UCB Pharma, and has served as a speaker for AbbVie, Amgen, Celgene, LEO Pharma, Merck, Novartis, Pfizer, and Union Therapeutics.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.html.
Additional information
Funding
References
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):1–10. doi: 10.1016/s0140-6736(20)31286-1.
- Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017. Br J Dermatol. 2021;184(2):304–309. doi: 10.1111/bjd.19580.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66. doi: 10.1038/jid.2014.325.
- Rønnstad ATM, Halling-Overgaard AS, Hamann CR, et al. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448–456.e430. doi: 10.1016/j.jaad.2018.03.017.
- Barrett A, Hahn-Pedersen J, Kragh N, et al. Patient-reported outcome measures in atopic dermatitis and chronic hand eczema in adults. Patient. 2019;12(5):445–459. doi: 10.1007/s40271-019-00373-y.
- Copley-Merriman C, Zelt S, Clark M, et al. Impact of measuring patient-reported outcomes in dermatology drug development. Patient. 2017;10(2):203–213. doi: 10.1007/s40271-016-0196-6.
- Williams HC, Schmitt J, Thomas KS, et al. The HOME core outcome set for clinical trials of atopic dermatitis. J Allergy Clin Immunol. 2022;149(6):1899–1911. doi: 10.1016/j.jaci.2022.03.017.
- de Bruin-Weller M, Biedermann T, Bissonnette R, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol. 2021;101(2):adv00402. doi: 10.2340/00015555-3751.
- de Bruin-Weller M, Deleuran M, Biedermann T, et al. The treat-to-target project in atopic dermatitis: one year on. Acta Derm Venereol. 2023;103:adv5382. doi: 10.2340/actadv.v103.5382.
- Leshem YA, Chalmers JR, Apfelbacher C, et al. Measuring atopic eczema symptoms in clinical practice: the first consensus statement from the harmonising outcome measures for eczema in clinical practice initiative. J Am Acad Dermatol. 2020;82(5):1181–1186. doi: 10.1016/j.jaad.2019.12.055.
- Leshem YA, Chalmers JR, Apfelbacher C, et al. Measuring atopic eczema control and itch intensity in clinical practice: a consensus statement from the harmonising outcome measures for eczema in clinical practice (HOME-CP) initiative. JAMA Dermatol. 2022;158(12):1429–1435. doi: 10.1001/jamadermatol.2022.4211.
- Silverberg J, Gooderham M, Katoh N, et al. Optimizing the management of atopic dermatitis with a new minimal disease activity concept and criteria and consensus-based recommendations for systemic therapy. Br J Dermatol. 2023;188(Supp 2):ljac140-022. doi: 10.1093/bjd/ljac140.022.
- RINVOQ. Prescribing information. North Chicago (Ill): AbbVie; 2022. [cited 2024 Feb 06]. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf.
- RINVOQ. Summary of Product Characteristics. AbbVie Deutschland GmbH & Co. KG; 2023 [cited 2023 April 24]. https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf.
- Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2(1):23. doi: 10.1186/s41927-018-0031-x.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi: 10.1016/s0140-6736(21)00588-2.
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413. doi: 10.1001/jamadermatol.2022.0029.
- Silverberg JI, de Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD up study results. J Allergy Clin Immunol. 2022;149(3):977–987.e914. doi: 10.1016/j.jaci.2021.07.036.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/s0140-6736(21)00589-4.
- He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20(2):181–192. doi: 10.1007/s40257-018-0413-2.
- Han Y, Woo YR, Cho SH, et al. Itch and Janus kinase inhibitors. Acta Derm Venereol. 2023;103:adv00869. doi: 10.2340/actadv.v103.5346.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519. doi: 10.1001/archderm.140.12.1513.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x.
- Foley C, Tundia N, Simpson E, et al. Development and content validity of new patient-reported outcome questionnaires to assess the signs and symptoms and impact of atopic dermatitis: the atopic dermatitis symptom scale (ADerm-SS) and the atopic dermatitis impact scale (ADerm-is). Curr Med Res Opin. 2019;35(7):1139–1148. doi: 10.1080/03007995.2018.1560222.
- Reich K, de Bruin-Weller MS, Deleuran M, et al. Higher levels of response on clinical atopic dermatitis severity measures are associated with meaningful improvements in patient-reported symptom and quality of life measures: integrated analysis of three upadacitinib phase 3 trials. J Eur Acad Dermatol Venereol. 2023;37(8):1634–1641. doi: 10.1111/jdv.18995.
- Silverberg JI, Simpson EL, Calimlim BM, et al. Determining severity strata for three atopic dermatitis patient-reported outcome questionnaires: defining severity score ranges for the worst pruritus numerical rating scale and the atopic dermatitis symptom and impact scales (ADerm-SS and ADerm-is). Dermatol Ther. 2022;12(12):2817–2827. doi: 10.1007/s13555-022-00836-5.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x.
- NIMH. Patient global impressions scale – Change, improvement, severity (PGI-C, PGI-I, PGI-S). 2023. https://eprovide.mapi-trust.org/instruments/patient-global-impressions-scale-change-improvement-severity.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi: 10.1111/j.1525-1470.2005.22303.x.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the national eczema association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012.
- Silverberg JI, Chiesa-Fuxench Z, Margolis D, et al. Epidemiology and burden of sleep disturbances in atopic dermatitis in US adults. Dermatitis. 2022;33(6S):S104–s113. doi: 10.1097/der.0000000000000731.
- Bawany F, Northcott CA, Beck LA, et al. Sleep disturbances and atopic dermatitis: relationships, methods for assessment, and therapies. J Allergy Clin Immunol Pract. 2021;9(4):1488–1500. doi: 10.1016/j.jaip.2020.12.007.

