Abstract
Objectives
Generalized pustular psoriasis (GPP) is a rare, life-threatening skin inflammatory disorder. This study aimed to describe the disease course, treatment strategies, and healthcare utilization among patients with GPP in Portugal.
Methods
This multicentric, observational, retrospective study included consecutive adult patients with GPP undergoing a dermatology evaluation in different reporting institutions by experienced dermatologists between 2002 and 2023.
Results
A total of 59 patients were assessed. Most of the cohort had a previous history of plaque psoriasis (71%) and 83% presented at least one comorbidity. At the initial encounter, 64% of the cohort needed hospitalization. Systemic involvement was common, including fever (37%), and elevated white blood cell count and erythrocyte sedimentation rate/C-reactive protein (49%). Nearly, 73% of patients initiated systemic drugs, and 70% had to discontinue the first treatment. During the study, 98% of patients experienced at least one flare. At the last visit, 3.4% of patients had died, and 71.2% exhibited signs of active disease despite undergoing treatment.
Conclusions
Our study demonstrates that GPP is a chronic, debilitating condition associated with systemic involvement, frequent flares, and hospitalizations, despite receiving multiple systemic treatments. Improved disease awareness and new treatments are needed to improve patient care and decrease the burden of the disease.
Introduction
Generalized pustular psoriasis (GPP) is a serious, immune-mediated inflammatory skin disease associated with significant morbidity and mortality (Citation1). It is a rare condition, with estimated prevalence ranging between 1.8 and 124 per million individuals (Citation2). The European consensus statement on phenotypes of pustular psoriasis (ERASPEN) defines GPP as the occurrence of macroscopically visible primary sterile pustules on nonacral skin, excluding psoriasis plaques (Citation3). However, due to the highly heterogeneous clinical presentation that can manifest at any age, diagnosing GPP poses a considerable clinical challenge (Citation3). Moreover, histological findings lack specificity, typically revealing subcorneal and intraepidermal pustules (Citation4). Consequently, diagnosis relies on history, morphologic features, and exclusion of secondary conditions. GPP exhibits a chronic course that varies significantly, presenting as acute flares of pustulation or a more persistent subacute form of the disease (Citation1,Citation5). The flares, potentially life-threatening, commonly manifest alongside systemic symptoms such as fever, malaise, and other extracutaneous manifestations (Citation5). Most flares last 2–5 weeks and frequently require hospitalization, imposing a substantial burden on patients (Citation6,Citation7). Notably, depression and anxiety are common, further exacerbating the patients’ compromised quality of life (Citation7,Citation8).
Given the rarity of GPP, there is a paucity of reported clinical characteristics among patients experiencing this disease in the literature. Additionally, limited disease awareness complicates the patient’s journey. Until recently, treatment strategies for GPP were primarily extrapolated from trials involving patients with plaque psoriasis with limited evidence for their clinical efficacy (Citation5,Citation9). Consequently, a comprehensive understanding of the clinical features and natural history of GPP, and how it responds to implemented treatment modalities is imperative to provide physicians with the necessary information for informed decision-making.
The objective of this multicenter study is to elucidate the clinical characteristics, triggering factors, morbidity, disease progression and treatment response in patients diagnosed with GPP in Portugal.
Material and methods
This is a multicentric, observational, retrospective cohort study involving patients with GPP from 9 Portuguese centers. The present study was conducted in accordance with the Declaration of Helsinki initially published in 1964 on Ethical Principles for Medical Research Involving Human Subjects and received approval from the local ethical committee.
Study design
In this retrospective multicentric study, we included patients (≥18 years of age) diagnosed with GPP between 2002 and 2023, undergoing a dermatology evaluation in the reporting institution with active pustular disease at the initial encounter. The clinical confirmation of GPP was performed by a dermatologist and was supported by histological examination when deemed necessary. Patients with other pustular diseases were excluded. No other strict exclusion criteria were applied to fulfill the study’s objectives, aiming to provide a descriptive analysis of an inclusive sample of all GPP cases observed in Portuguese routine clinical practice.
Data collection
The initial visit marked the first date of active disease evaluation in the reported institution (emergency department or outpatient setting). Demographic information, past medical history, comorbidities, family history, triggering factors and disease course (including all episodes in the reporting institution until 2023) were extracted from medical records. Data were collected using a shared case report form completed by a selected group of dermatologists with recognized experience in GPP diagnosis. Flares of GPP were defined as the acute appearance of pustules affecting large body areas on an erythematous skin, accompanied or not by systemic symptoms, such as fever, leukocytosis, and fatigue. Whenever available, data on disease extension was estimated by proportion of the body surface area involvement (BSA %) and detailed for body regions, including mucosal and nail involvement. Data on Dermatology life quality index (DLQI) questionnaire were also extracted whenever available (Citation10).
Statistical analysis
Descriptive statistics were used to summarize patient and disease-related information. Continuous variables were presented as mean ± standard deviation or as median (interquartile range), while categorical variables were expressed as proportions. The Wilcoxon test was employed to compare continuous, paired variables. Statistical analysis was performed using the IBM Statistical Package for the Social Sciences software, version 24 (IBM Corporation), with a p value of < 0.05 considered to be statistically significant.
Results
Fifty-nine patients met the inclusion criteria and were followed in the reporting center for a median period of 3.5 (range 0–40) years. Upon initial presentation, most of the patients (66.1%, n = 39) presented at the emergency department, whereas 33.9% (n = 20) were assessed in an outpatient setting. Eighty-five percent of patients (n = 50) had no history of GPP and 71.2% (n = 42) had a prior history of plaque psoriasis. The demographic characteristics of the sample are presented in .
Table 1. Demographic characteristics of the sample (n = 59).
Comorbidities
More than eighty percent of the sample (n = 49, 83.1%) had at least one comorbidity. The detailed associated conditions and lifestyle habits are detailed in .
Table 2. Comorbidities and lifestyle habits reported in the initial visit (n = 59).
GPP potential precipitating factors
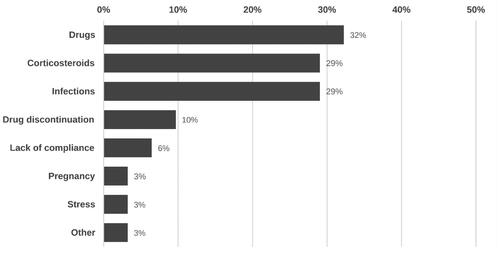
In 52.5% of patients (n = 31), at least one suspected trigger for active disease was identified. The most recorded inciting factor was drugs, followed by suspension of systemic corticosteroids, and infections (). Among the suspected associated drugs were ustekinumab, amoxicillin, terbinafine, nonsteroidal anti-inflammatory drugs, metformin, azathioprine, and itraconazole.
Clinical presentation
Sixty-four percent of patients (n = 38) were hospitalized during the initial encounter, and 18.6% (n = 11) presented with infections. During the initial evaluation, most patients reported pruritus (72.9%, n = 43) and skin pain (69.5%, n = 41), with 22% (n = 13) reporting joint pain. Additionally, 37.3% (n = 22) experienced fever and 16.9% (n = 10) exhibited tachycardia. Mucous membrane involvement was reported in a minority of patients (3.4%, n = 2), and nail involvement was noted in15.3% (n = 9).
Regarding laboratory abnormalities, an increased white blood cell count, and elevated erythrocyte sedimentation rate/C-reactive protein were found in 49.2% (n = 29), hepatic abnormalities in 10.2% (n = 6), kidney abnormalities 8.5% (n = 5), and low calcium levels in 6.8% (n = 4).
Treatment
At the initial encounter, 27.1% of patients (n = 16) were treated with topical agents, 25.4% (n = 15) with retinoids, 22.0% (n = 13) with cyclosporin,15.3% (n = 9) with systemic steroids, 6.8% (n = 4) with methotrexate, 1.7% (n = 1) with dapsone and 1.7% (n = 1) with secukinumab. The first treatment course had a mean duration of 6 (range 1–120) months. Adverse events were reported for 20.3% of patients (n = 12) and the first treatment had to be discontinued in 69.5% (n = 41): 58.5% (n = 24) of those due to lack of efficacy, 14.6% (n = 6) due to adverse events, and 24.4% (n = 10) due to the risk of toxicity. At least a second systemic treatment course was reported for 61.0% of the patients (n = 36), a third treatment course for 33.9% (n = 20), a fourth for 13.6% (n = 8) and a fifth treatment had to be tried in 5.1% (n = 3) of the patients. In total, 15 different systemic treatments were recorded during the study period. The systemic treatment details are detailed in .
Table 3. Systemic treatment option during the study period, duration of treatment cycle (months), reported adverse events and treatment discontinuation.
Clinical course
During the study interval, 98.3% of patients (n = 58) experienced at least one flare, with 72.9% (n = 43) having 1–2 flares, 10.2% (n = 6) experiencing 3–4 flares and 15.3% (n = 9) having more than 4 flares.
Median BSA was available for a subsample of 25 patients and varied from 20 (31) at the first evaluation to 15 (18) at the last visit, which was a significant decrease (p < 0.001). Median DLQI was available for a subsample of 26 patients and changed from 20 (10) at baseline to 10 (7) at last visit (p < 0.001).
At the last visit, 5.1% (n = 3) of patients had died (3.4% (n = 2) due to GPP, 1.7% (n = 1) due to other causes), 1.7% (n = 1) was experiencing a flare, 71.2% (n = 42) exhibited signs of active disease despite undergoing treatment and, for the remaining patients, follow-up was lost, or no status information was available.
Healthcare utilization
During the study period, a total of 40 patients (67.8%) were hospitalized at least once due to GPP symptoms, and 20% (n = 12) had more than two hospitalizations, with a mean (SD) rate of 0.33 ± 0.44 hospitalizations per year. Nearly 90% (n = 53) of the patients had at least one visit to the emergency room, while 23.7% (n = 14) had at least three times. Further details are presented in .
Table 4. Healthcare utilization during the study period in patients with generalized pustular psoriasis (n = 59).
Discussion
In this multicentric study, we describe the clinical course and disease burden of patients with GPP treated in Portuguese healthcare centers. Despite several treatment attempts, most patients in our study experienced at least one flare and most patients required at least one hospitalization and/or an emergency department visit. Our findings support that GPP, albeit rare, manifests as a heterogeneous, chronic, severe, and debilitating disease, imposing a substantial burden on patients and healthcare systems, necessitating a more tailored and comprehensive treatment approach.
In our study, GPP predominantly affected female patients (66%) with a mean age at presentation of 55.1 ± 21.3 years, consistent with prior literature sources (Citation1,Citation11,Citation12). The majority of the cohort exhibited a history of plaque psoriasis (71%), also aligned with prevalence ranges of 30-70% reported in the literature (Citation11,Citation12). Previous studies report plaque psoriasis typically antedates GPP by several years, a pattern also confirmed by our data (Citation11,Citation12). Notably, 15% of the sample had a previous history of psoriatic arthritis, a proportion similar to that reported by Mastacouris et al. and Okubo et al. (Citation7,Citation13) Importantly, no patient in our sample had a family history of pustular psoriasis, and disease awareness was possible low. This highlights the pivotal role of patient education, particularly in distinguishing the GPP from plaque psoriasis (Citation14).
GPP flares may arise spontaneously or can be linked with potential triggering factors (Citation4,Citation5,Citation11,Citation15). While multiple triggers have been associated with GPP flares, evidence supporting the specific role of these factors remain scarce. In our cohort, a potential triggering factor was identified for most patients, with steroids (29%), other drugs (32%), and infections (29%) being the most common ones.
The clinical course of GPP is highly variable, from a more chronic form to a relapsing course, with flares differing in both severity and frequency. Most patients report pruritus and pain and this was also verified in our cohort(Citation1). In contrast to plaque psoriasis, acute flares of GPP frequently involve systemic inflammation and extracutaneous manifestations that demand urgent management (Citation7,Citation16). In our study, more than 60% of the patients required hospitalization and concurrent infections were not rare (19%). A significant proportion reported articular pain (22%) and signs of systemic involvement, including fever (37%), tachycardia (17%), elevated white blood cell counts and elevated erythrocyte sedimentation rate/C-reactive protein (49%). Consequently, GPP is a systemic disease with a high burden of illness and life-threatening potential. Early recognition, multilevel support and attempted intervention and treatment are crucial to prevent further deterioration (Citation1,Citation4,Citation14). Moreover, comorbidities were highly prevalent in our cohort (more than 80% of the patients presented with at least one comorbidity), adding complexity to disease management, and prompting a multidisciplinary, holistic approach (Citation4).
The rarity of GPP poses challenges in conducting clinical trials, and treatment recommendations are typically based on insufficient evidence (Citation17). The treatment strategy for GPP often mirrors that of severe cases of plaque psoriasis, yet evidence regarding its efficacy remains scarce (Citation18). Recent evidence supports that GPP is a genetically and phenotypically distinct entity from plaque psoriasis, exhibiting a heavier systemic burden and requiring tailored treatment strategies that address its unique characteristics (Citation7). In our study, most patients (73%) needed systemic treatment, immunosuppression or steroids were the most common first choice, followed by retinoids. While systemic steroids have been discouraged due to potential rebound flares, they are frequently used in the acute control of the disease. Systemic retinoids, and immunosuppressants (methotrexate and cyclosporine) have been widely used in GPP flares, albeit being limited by toxicity and slow onset of response. Notably, in our study, the first treatment had to be discontinued in 70% of patients due to lack of efficacy, adverse events or risk of toxicity. At least a second systemic treatment cycle was reported for 61% of patients, a third for 34%, a fourth for 14% and a fifth attempt of a different systemic treatment had to be tried in 5%. In total, 15 different systemic treatments were attempted. Despite all these attempts and different interventions, the disease was not evidence adequately controlled: median BSA significantly improved, but it still scored 15 (Citation18) % at the last visit. The median DLQI of 10 (Citation7), documented during the last follow-up visit, also underscores the substantial impact of GPP on patient’s quality of life despite all the treatment attempts. Moreover, throughout the study period, 98% of patients experienced at least one flare and the majority of patients required at least one hospitalization (68%), with an average rate of 0.54 ± 0.52 emergency department visits per year. This heightened healthcare utilization reflects inadequate disease control using current treatment strategies. This includes classic biologic agents, that were tried in 30 patients in our study. Although the small sample size of our study does now allow for specific subgroup comparisons, the fact that none of the utilized agents targets any specific aspect of GPP pathogenesis may contribute to the frequent treatment failures (Citation19). Unlike plaque psoriasis, research has shown that GPP is driven by an inflammatory response resulting from hyperactivation of innate immunity primarily involving the IL-36 axis (Citation20,Citation21). Emerging therapies targeting IL-36R, such as spesolimab and imsidolimab, show promising results in terms of efficacy and safety, offering potential improvements in GPP management (Citation19).
This study has inherent limitations due to its retrospective design. Information on some parameters was not available for all patients, and visits were not standardized within strict time frames for treatment response assessment. Available data did not allow us to estimate important measurements of disease activity and severity, including the Generalized Pustular Psoriasis Area and Severity Index (GPPASI) and the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA), and further prospective studies addressing these measurements may improve our understanding of the clinical course of GPP. Additionally, the relatively short follow-up period limited the evaluation of additional causative factors and details on treatment response. Triggering factors were reported in medical records, and future studies focusing on causality and temporal association may clarify their role.
In conclusion, our analysis highlights the chronic, heterogeneous, and debilitating nature of GPP. Flares of GPP can be severe, leading to multiple hospitalizations and emergency department visits, significantly compromising the quality of life and survival of patients. Standard treatment options often fail to achieve adequate disease control, and multiple attempts are often needed. Appropriate patient awareness and diligent disease management have the potential to reduce healthcare visits, and the overall burden of the disease for patients and healthcare systems.
Author contributions statement
Concept and design: TT, JA, AB, JA, DB, JR, DS, CC, AGP, AAM, JFM, BVG, PP, GMP, PQ, FMB, LT, SM, MJPL, HO, PV, JTS, PF, RTB
Analysis and interpretation of data: TT, JA, LT
Drafting of the manuscript: TT, JA
Critical revision of the manuscript for important intellectual content: TT, JA, AB, JA, DB, JR, DS, CC, AGP, AAM, JFM, BVG, PP, GMP, PQ, FMB, LT, SM, MJPL, HO, PV, JTS, PF, RTB
Final approval: TT, JA, AB, JA, DB, JR, DS, CC, AGP, AAM, JFM, BVG, PP, GMP, PQ, FMB, LT, SM, MJPL, HO, PV, JTS, PF, RTB
All authors agree to be accountable for all aspects of the work.
Disclosure statement
Tiago Torres has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius Kabi, Janssen, LEO Pharma, Eli Lilly, MSD, Mylan, Novartis,
Pfizer, Samsung-Bioepis, Sanofi-Genzyme, Sandoz and UCB.
Pedro Ponte has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by Leo, Janssen, Abbvie, Novartis, Pfizer, Lilly, and Almirall.
Gabriela Marques Pinto has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by Abbvie, Almirall, Janssen, LEO Pharma, Lilly, and Novartis.
Maria João Paiva Lopes has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Almirall, Boehringer Ingelheim, Janssen, LEO Pharma, Eli Lilly, Mylan, Novartis, Pfizer, Sanofi-Genzyme, and Viatris.
The remaining authors have no conflicts of interest.
Additional information
Funding
References
- Rivera-Díaz R, Daudén E, Carrascosa JM, et al. Generalized pustular psoriasis: a review on clinical characteristics, diagnosis, and treatment. Dermatol Ther (Heidelb). 2023;13(3):1–7. doi: 10.1007/s13555-022-00881-0.
- Armstrong AW, Elston CA, Elewski BE, et al. Generalized pustular psoriasis: a consensus statement from the national psoriasis foundation. J Am Acad Dermatol. 2024;90(4):727–730.
- Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1792–1799. doi: 10.1111/jdv.14386.
- Puig L, Choon SE, Gottlieb AB, et al. Generalized pustular psoriasis: a global delphi consensus on clinical course, diagnosis, treatment goals and disease management. J Eur Acad Dermatol Venereol. 2023;37(4):737–752. doi: 10.1111/jdv.18851.
- Choon SE, Lebwohl MG, Turki H, et al. Clinical characteristics and outcomes of generalized pustular psoriasis flares. Dermatology. 2023;239(3):345–354. doi: 10.1159/000529274.
- Löfvendahl S, Norlin JM, Ericson O, et al. Prolonged sick leave before and after diagnosis of generalized pustular psoriasis: a eneral population-based register study. Acta Derm Venereol. 2023;103:adv6497. doi: 10.2340/actadv.v103.6497.
- Okubo Y, Kotowsky N, Gao R, et al. Clinical characteristics and health-care resource utilization in patients with generalized pustular psoriasis using real-world evidence from the enerali medical data center database. J Dermatol. 2021;48(11):1675–1687. doi: 10.1111/1346-8138.16084.
- Patel PM, Sanchez-Melendez SN, Nambudiri VE. A narrative review of studies assessing the quality of life in patients with generalized pustular psoriasis. Exp Dermatol. 2023;32(8):1227–1234. doi: 10.1111/exd.14787.
- Maçães CO, Lé AM, Torres T. Generalized pustular psoriasis: the new era of treatment with IL-36 receptor inhibitors. J Dermatolog Treat. 2022; Oct 333(7):2911–2918. doi: 10.1080/09546634.2022.2089335.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x.
- Bellinato F, Gisondi P, Marzano AV, et al. Characteristics of patients experiencing a flare of generalized pustular psoriasis: a multicenter observational study. Vaccines (Basel). 2023;11(4):740. doi: 10.3390/vaccines11040740.
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: ANALYSIS of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53(6):676–684. doi: 10.1111/ijd.12070.
- Mastacouris N, Feda A, Strunk A, et al. Risk factors for generalized pustular psoriasis: a case-control study. J Am Acad Dermatol. 2023;89(4):846–848. doi: 10.1016/j.jaad.2023.06.018.
- Balato A, Ambrogio F, Burlando M, et al. Commentary: unmet needs in generalized pustular psoriasis in clinical practice. Dermatol Ther (Heidelb). 2023;14(1):5–13. doi: 10.1007/s13555-023-01073-0.
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(Suppl 1):21–29. doi: 10.1007/s40257-021-00654-z.
- Hanna ML, Singer D, Bender SD, et al. Characteristics of hospitalizations and emergency department visits due to generalized pustular psoriasis in the United States. Curr Med Res Opin. 2021;37(10):1697–1703. doi: 10.1080/03007995.2021.1951192.
- Fujita H, Terui T, Hayama K, et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 2018;45(11):1235–1270. doi: 10.1111/1346-8138.14523.
- Abduelmula A, Rankin BD, Sood S, et al. Management of adult generalized pustular psoriasis using biologics: a systematic review. J Am Acad Dermatol. 2023;89(2):417–419. doi: 10.1016/j.jaad.2023.04.031.
- Bernardo D, Thaçi D, Torres T. Spesolimab for the treatment of generalized pustular psoriasis. Drugs [Internet]. 2023;84(1):45–58. doi: 10.1007/s40265-023-01988-0.
- Capon F. A viewpoint on the genetic determinants of eneralized pustular psoriasis. Exp Dermatol. 2023;32(8):1188–1193. doi: 10.1111/exd.14746.
- Hawkes JE, Visvanathan S, Krueger JG. The role of the interleukin-36 axis in generalized pustular psoriasis: a review of the mechanism of action of spesolimab. Front Immunol. 2023;14(vember):1292941. doi: 10.3389/fimmu.2023.1292941.