Abstract
Purpose: Evidence on treatment preferences of patients with moderate-to-severe atopic dermatitis (AD) in the United States (US) is limited and an assessment of treatment preferences in this group is warranted.Materials and methods: An online discrete choice experiment survey was conducted (June 2023) among US adults with self-reported moderate-to-severe AD or experience with systemic therapy who had inadequate response to topical treatments. Preference weights estimated from conditional logistic regression models were used to calculate willingness to trade off and attributes’ relative importance (RI).Results: Participants (N = 300; mean age: 45 years; 70% females; 52% systemic therapy experienced) preferred treatments with higher efficacy, lower risk of adverse events (AEs), and less frequent blood tests (p < .05). Treatment attributes, from high to low RI, were itch control (38%), risk of cancer (23%), risk of respiratory infections (18%), risk of heart problems (11%), sustained improvement in skin appearance (5%), blood test frequency (3%), and frequency and mode of administration (2%); together, AE attributes accounted for more than half of the RI.Conclusions: Participants preferred AD treatments that maximize itch control while minimizing AE risks, whereas mode of administration had little impact on preferences. Understanding patients’ preferences may help improve shared decision-making, potentially leading to enhanced patient satisfaction with treatment, increased engagement, and better clinical outcomes.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease characterized by pruritic, erythematous, and scaly skin lesions (Citation1). AD poses a negative impact on mental health, sleep, work productivity, and daily activities and is associated with an increased risk of other comorbidities (e.g., asthma, hay fever, food allergy, and eosinophilic esophagitis) (Citation1–5).
Different treatment solutions are needed for individualized management of AD. Current options for managing moderate-to-severe AD include topical emollients, systemic therapies, and biologics, which are often used in combination (Citation6). In recent years, monoclonal antibodies and Janus kinase (JAK) inhibitors have become available (Citation6–10). These systemic therapies, when used together with topical corticosteroids, are effective at controlling clinical signs and AD-related symptoms, but they offer varied levels of efficacy, safety, response times, and frequency and mode of administration (Citation11,Citation12). Some AD treatments may offer better efficacy (e.g., itch reduction or improvement in skin appearance) but associate with a higher risk of severe adverse events (AEs) (Citation13,Citation14). Furthermore, efficacy of various therapies could be difficult to interpret, as assessments of clinical endpoints at week 16 in some clinical trials may favor rapid-acting therapies while disfavoring those with slower onset (Citation7,Citation8). Treatments can also differ in their modes of administration (oral, injectable) and frequency of administration (once daily vs. twice monthly vs. once monthly).
Understanding the degree to which different treatment attributes are valued by patients may help improve discussions between patients and physicians around treatment choices. Discrete choice experiments (DCEs) are used to quantify treatment preferences and the tradeoffs patients are willing to make between the benefits and risks of various treatment options. Prior studies on patient preferences for AD treatments primarily focus on countries outside of the United States (US), and existing US studies are limited in scope (Citation4,Citation13,Citation15–18). Given differences in cultural and healthcare environments that may affect patient preferences for AD treatments, a comprehensive DCE study in the US is warranted. Furthermore, patients with other chronic diseases prefer oral treatments over injectables; however, evidence on patient preference for the mode and frequency of administration for AD treatments is lacking, and a better understanding of these preferences may help improve drug adherence (Citation15,Citation19). The purpose of this DCE study was to better understand treatment preferences for patients with moderate-to-severe AD in the US with a focus on different treatment attributes of monoclonal antibodies and JAK inhibitors.
Methods
Data source and study population
Participants were recruited from a panel of a well-established market research firm, Dynata, through email invitations. Data collection for the online DCE survey was conducted in June 2023. Eligible participants were adults (18 years of age or older) who had been diagnosed with AD for at least 1 year, with self-reported moderate-to-severe AD or experience with systemic therapy and had experienced inadequate response to topical treatments.
Study design and measures
This study was conducted in two phases: the first phase (qualitative) looked at attributes of importance for treatment decisions and were identified based on a literature review, clinical opinion, and qualitative interviews with eligible participants; in the second phase (quantitative), a web-based survey was conducted to assess and quantify preferences for AD treatment attributes among eligible participants using a DCE. The DCE portion of the study was conducted in accordance with the recommendations of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Research Practices for Conjoint Analysis Task Force (Citation20,Citation21). The survey consisted of screening questions to confirm participants’ eligibility, followed by a DCE component to assess participants’ preferences for different AD treatment options, and ended with questions about participant demographic and clinical characteristics.
Phase I: identification of attributes and levels
In this qualitative phase, a total of 17 attributes of potential importance to participants when making a treatment decision were first identified based on a literature search and data from clinical trials, with a focus on attributes which represent differentiating features between monoclonal antibodies and JAK inhibitors (Citation7–9,Citation14,Citation22–24). Semi-structured, one-on-one qualitative interviews were conducted with ten eligible participants with moderate-to-severe AD to understand which treatment attributes were most important when making treatment decisions (Citation25). Based on these qualitative interviews and input from clinical experts, the following attributes were included in the DCE in Phase II: sustained improvement in skin appearance, probability of achieving meaningful itch control, risk of respiratory infections, risk of cancer, risk of heart problems, frequency and mode of administration, and blood test frequency (Supplemental Table 1).
Phase II: DCE survey
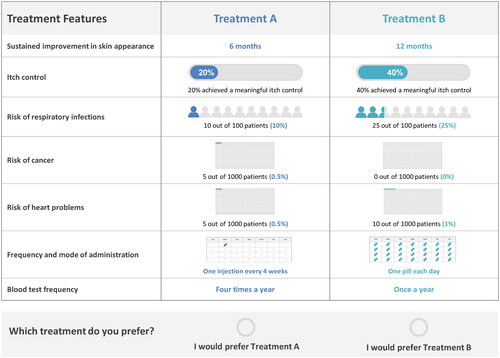
Based on the attributes and levels identified in Phase I, 40 choice tasks were generated using a D-efficient design in Ngene (a software specifically developed for DCEs) (Citation26). The choice tasks were divided into four blocks with ten tasks to limit the response burden. Each participant was randomly assigned to one of the blocks during the survey. Two additional choice tasks were presented to each participant to assess the internal validity of the responses (including a dominance test where all attributes in one profile were superior to those in the other profile, and a stability test repeating one of the previous choice tasks (Citation27)). Altogether, each participant was presented twelve choice tasks, each containing two hypothetical AD treatment profiles with combinations of various attribute levels. For each choice task, participants were asked to choose the treatment they preferred between the two options ().
Statistical analyses
Descriptive statistics
Participants’ demographic and clinical characteristics were descriptively summarized. Means, medians, and standard deviations were reported for continuous variables; frequency counts and percentages were reported for categorical variables.
Assessment of preferences for treatment attributes
Preference data collected from the DCE were analyzed using a conditional logistic regression model, in which the dependent variable was the participant’s preference data for a given choice task (i.e., a binary variable indicating if a given treatment option was selected), and the independent variables were the levels of each attribute. Efficacy and risk attributes were evaluated as continuous variables, while mode and frequency of administration as well as blood test frequency were evaluated as categorical variables. Coefficients obtained from regression analyses showed the preference weights of the attributes, with the associated p values indicating whether the estimated coefficient was statistically different from zero. The coefficients were used to calculate the willingness to trade off (WTT) to estimate the percentage points in the chance of achieving itch control a participant would be willing to forego to receive a treatment with a reduction of 1 percentage point in the risk of a particular AE.
Part-worth utility, which measures the utility associated with each attribute level, was calculated by multiplying the coefficients with each potential level of a given attribute. The relative importance (RI) of attributes was computed by multiplying the coefficients with the difference between the best and worst levels of the corresponding attribute and then normalizing them to percentages; this allowed for comparisons across treatment attributes (Citation28).
Subgroup analyses
To understand potential variations in preferences by treatment experience, exploratory analyses were also conducted in two subgroups based on whether participants had experience with systemic therapy (i.e., participants with systemic treatment experience and without systemic treatment experience). Information on systemic therapy experience was based on survey questions asking participants to select all treatments they had used to manage their AD; participants who selected oral pills or injectable treatments were considered to have experience with systemic therapy. The target sample size for each subgroup was approximately half of that of the overall sample.
Sensitivity analyses
Sensitivity analyses were conducted to evaluate the internal validity of the preference data. Based on the results of the two choice tasks designed to assess data quality, participants who failed either validity test (i.e., dominance or stability test) were excluded from the sensitivity analyses. All analyses were performed using the SAS Enterprise Guide statistical software version 7.1 (SAS Institute, Cary, NC).
Results
Participant characteristics
A total of 300 participants completed the survey and were included in the overall sample. Among all participants, 70.0% were females, 78.0% were White or Caucasian (11.0% Black, 4.7% Asian, 3.7% mixed, 2.7% other), 86.0% weighed less than 100 kg (220 lbs), and there was good coverage of all US regions (). Over half were employed (66.0%) and had a bachelor’s degree or above (60.6%).
Table 1. Participant demographics and clinical characteristics.
In terms of clinical characteristics, 50.0% of the participants had experienced severe symptoms at some point since their AD diagnosis, and around half (49.7%) received their first AD treatment five or more years ago (). The majority (80.0%) received topical treatment, 31.0% received oral therapies, and 14.7% received injectable therapies at the time of survey completion. Most participants (87.8%) were affected by AD on visible body regions (i.e., face, neck, hand, feet) as well as other parts of the body (57.7%).
Participants with systemic therapy experience (ever) accounted for 52.0% of the sample (48.0% without systemic therapy experience). Participants without systemic therapy experience were more likely to be females than those with systemic experience (80.6% and 60.3%, respectively). They were also more likely to have received their first AD treatment at least five years ago (59.0% and 41.0%, respectively) and to have experienced less severe symptoms (both at the time of survey completion and at any time) than those with systemic experience.
DCE results
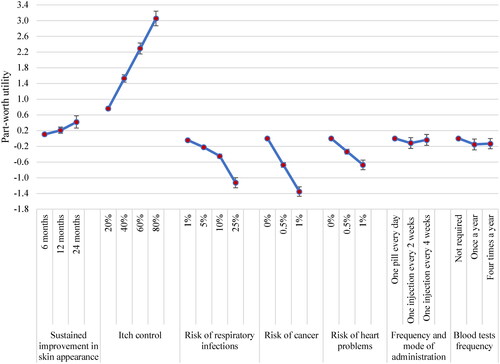
Based on the conditional logistic regression model, participants demonstrated a strong preference for all DCE attributes (p < .05), except for frequency and mode of administration (). Positive preference weights associated with sustained improvement in skin appearance and itch control indicated that participants preferred treatments with higher efficacy. As evidenced by the negative preference weights, participants preferred treatments with a lower risk of AEs, with a particularly strong preference to avoid risk of cancer. Regarding monitoring requirements, having no required blood tests was preferred to having blood tests once a year or four times a year (). Frequency and mode of administration did not have a statistically significant impact on preferences.
Table 2. Preference weights and willingness to trade off in the overall sample.
Participants were willing to trade off varying chances of achieving meaningful itch control to avoid AE risks. On average, participants were willing to trade off 35.4, 17.7, and 1.2 percentage points in the chance of achieving itch control to avoid 1 percentage point risk of cancer, heart problems, and respiratory infections, respectively (). The sensitivity analysis, excluding 81 (27%) participants who failed either of the validity tests, had similar results and supported the robustness of our analysis (Supplemental Table 2).
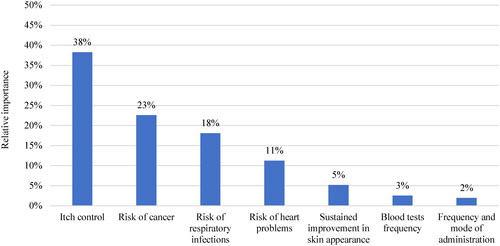
Part-worth utility showed the utility associated with each attribute level. Part-worth utilities increased with longer sustained improvement in skin appearance and a higher chance of achieving meaningful itch control, whereas higher risks of AEs were associated with disutility (). Participants valued the probability of attaining itch control more than sustained improvement in skin appearance. When considering the range of attribute levels included in the DCE design, itch control (RI: 38%) was ranked as the most important attribute, followed risk of cancer (RI: 23%), risk of respiratory infections (RI: 18%), and risk of heart problems (RI: 11%); the RI of all these AE attributes combined (i.e., 52%) was higher than that of itch control. Sustained improvement in skin appearance (RI: 5%), blood test frequency (RI: 3%), and frequency and mode of administration (RI: 2%) were the least important attributes ().
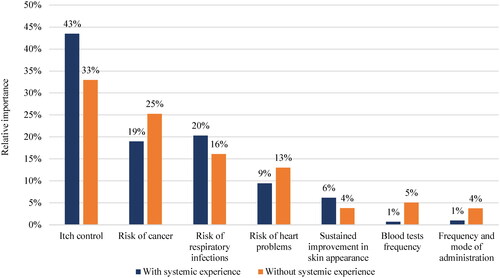
Consistent with the overall sample, all efficacy and AE attributes impacted preferences across both those with and without systemic therapy experience (). However, blood test frequency as well as frequency and mode of administration only had a statistically significant impact on preferences among participants without systemic therapy experience. Specifically, these patients prefer avoiding blood tests and injections every two weeks. The preference for injection every four weeks was not statistically different than that from a daily oral pill, indicating that participants without systemic therapy experience may be opposed to an injection every two weeks, but if injection frequency was reduced to every four weeks, this would not negatively impact their treatment preference.
Table 3. Preference weights and willingness to trade off stratified by systemic experience.
Itch control ranked highest in both those with and without systemic exposure (RI: 43% [with systemic experience]; RI: 33% [without systemic experience]), followed by all AE attributes, generally consistent with rankings in the overall sample (). However, RI for serious AEs (i.e., risks of cancer and heart problems) were perceived as more important among participants without systemic experience than those with systemic experience. Monitoring and treatment administration attributes ranked lowest in both groups, as in the overall sample.
Discussion
Given the rapidly evolving treatment landscape for moderate-to-severe AD, this study investigated patient preferences for different treatment attributes of newer systemic therapies in the US, namely monoclonal antibodies and JAK inhibitors. Patients with moderate-to-severe AD preferred treatments that maximized itch control while minimizing the risks of AEs (in decreasing order of importance: cancer, respiratory infections, heart problems), whereas frequency and mode of administration had little impact on patient preferences. Sustained improvement in skin appearance, blood test frequency, and frequency and mode of administration were the least important attributes (in decreasing order of importance). Patient preferences also varied based on experience with systemic therapy, which may be driven by differences in severity across groups. Patients without systemic therapy experience were willing to trade off more efficacy (chance of achieving meaningful itch control) to avoid risks of serious AEs (i.e., risks of cancer and heart problems) and considered these AEs more important (based on RI) than patients with systemic experience.
While only a limited number of prior studies assessed preferences in US patients with moderate-to-severe AD, our findings are generally consistent with those from previous DCE studies. For example, similar to our results, itch reduction was the most important attribute for adult patients with moderate-to-severe AD in the US (Citation13). In another DCE study conducted among patients with moderate-to-severe AD in France, Spain, and the UK, itch reduction was the most important attribute when making treatment decisions, whereas another efficacy attribute of improvement in skin appearance was relatively less important; similar trends were obtained in the gender subgroups (Citation15). Further research is needed to understand potential differences in preferences for specific AD treatment attributes among subgroups (e.g., females vs males) in the US.
Our study further refined the categorization of AEs to differentiate preferences across AE types; that is, each AE was considered separately. By contrast, other DCEs have considered AEs pooled together, and side effects were the most important attribute. For instance, in a DCE study conducted among moderate-to-severe AD patients in Japan, minimizing the risk of mild side effects was the most important treatment attribute (Citation16). Similarly, another DCE study in Denmark, France, the UK, and Canada found that avoiding risk of severe AEs was the most important treatment attribute (Citation18). In our study, while the importance of each AE taken separately was lower than that of itch control, the overall importance of AEs, when considered together, is higher than that of itch control.
In contrast to our study, mode of administration can be an important treatment attribute, where patients preferred daily oral pills to a subcutaneous injection every two weeks; however, preference for injections every four weeks has not been assessed (Citation17,Citation18,Citation29). While frequency and mode of administration did not have a significant impact on patient preferences in the overall sample, patients without prior systemic experience would prefer a daily oral pill to an injection every two weeks, but would be indifferent between a daily pill and an injection every four weeks. Considering this, the option to have an injection every four weeks could be seen as a more convenient option from the patient perspective among systemic-naïve patients, and these patients may be more open to this option as an alternative to oral therapy. Although not included in our study, less frequent injections may also be associated with lower costs, which in turn could be valued by patients. Further research is warranted to better understand the impact of costs on patient preferences.
Understanding patients’ willingness to trade off efficacy and safety may help shared treatment decision-making, enhance treatment satisfaction, and foster improved adherence to treatment. Participants with moderate-to-severe AD prefer treatments that offer meaningful itch reduction and minimize safety risks, reflecting the real-world burden experienced by patients with AD. Despite half of the patients having used systemic therapy, and 90% on current treatment, most patients still experienced moderate or severe symptoms and had involvement in visible areas, such as face, neck, and hand. Safe and effective therapies are needed for moderate-to-severe AD. Physicians and patients should consider the risk of AEs - especially serious AEs, such as malignancy and heart problems - associated with certain treatments when making treatment decisions, by carefully considering the implications on patients’ adherence to treatment and related quality of life, as well as the impacts on the healthcare system more generally.
Limitations
First, the current study included only respondents who agreed to participate in this study. Although the study sample included participants from all US regions, it might differ from the broader US population with moderate-to-severe AD. Similarly, since convenience sampling was used, selection bias may exist in the resulting sample of respondents. Comparing the current participant demographics with that reported in a US national survey of AD (Citation30), participants in this study appeared to be slightly younger, more educated, and had a numerically higher proportion of female and White race than those with moderate-to-severe AD captured in the national survey; these demographic characteristics may reflect the better access to and greater willingness to participate in online surveys among the vendor’s panel. Second, this study relied on respondents’ recollection of past events. Recall bias, or errors in the accuracy or completeness of respondents’ recalled experiences can be a limitation, mainly if more recent events influence memories. This study aimed to minimize recall bias by asking respondents to reflect on events that occurred in the recent past. Relatedly, due to the self-reported nature of the study, participant characteristics such as AD diagnosis and severity could not be confirmed. Third, additional attributes may have been important to inform patients’ preferences; however, considering the response burden, only a few key attributes could be included in the DCE questions. Key attributes were identified using a three-pronged data-driven approach through targeted literature review, expert clinical input, and qualitative interviews, with the goal to include attributes most important to patients with moderate-to-severe AD. Fourth, information on participants’ treatment experience was collected for broad treatment categories and not specific therapies; as such, impact of individual therapy experience could not be delineated. Fifth, conditional logit model is subject to limitations such as the assumption of preference homogeneity; exploration of preference heterogeneity warrants future research. Finally, overall means may not reflect the preferences of individual patients.
Ethics statement
This study was approved by the Western Copernicus Group Institutional Review Board (WCG IRB) and exempt from continuing review by the Board under the revised common rule. The research met the requirements for a waiver of documentation of consent under 45 CFR §46.117(c)(1)(ii).
Author contributions
AG, MGL, NCH, YM, and KG contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. SRF, AJC, and SB contributed to study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Previous presentations
Part of the materials was presented at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference in May 2023 and at the 43rd Fall Clinical Dermatology Conference in October 2023.
Supplemental Material
Download MS Word (45.8 KB)Acknowledgments
Medical writing assistance was provided by professional medical writer, Flora Chik, PhD, an employee of Analysis Group, Inc., and was funded by LEO Pharma A/S, which is the manufacturer of tralokinumab.
Disclosure statement
SRF has received research, speaking and/or consulting support from Eli Lilly and Company, GlaxoSmithKline/Stiefel, AbbVie, Janssen, Alovtech, vTv Therapeutics, Bristol-Myers Squibb, Samsung, Pfizer, Boehringer Ingelheim, Amgen, Dermavant, Arcutis, Novartis, Novan, UCB, Helsinn, Sun Pharma, Almirall, Galderma, LEO Pharma, Mylan, Celgene, Ortho Dermatology, Menlo, Merck & Co, Qurient, Forte, Arena, Biocon, Accordant, Argenx, Sanofi, Regeneron, the National Biological Corporation, Caremark, Teladoc, BMS, Ono, Micreos, Eurofins, Informa, UpToDate and the National Psoriasis Foundation. He is founder and part owner of Causa Research and holds stock in Sensal Health; AC, and SB are employees of LEO Pharma; AG, MGL, NCH, and YM are employees of Analysis Group and have received consulting fees from LEO Pharma Inc.
Data availability statement
The data that support the findings of this study are available on reasonable request. The data are not publicly available and cannot be deposited into a public repository, primarily due to the fact that study participants did not consent to this. The project team was responsible for not compromising research participant privacy/consent.
Additional information
Funding
References
- Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017. Br J Dermatol. 2021;184(2):1–10. doi:10.1111/bjd.19580.
- Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86(1):35–42.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–151. doi:10.1016/j.anai.2019.04.020.
- Carrascosa Carrillo JM, Baselga Torres E, Gilaberte Calzada Y, et al. Quantifying physician preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. Dermatol Ther. 2022;12(5):1197–1210. doi:10.1007/s13555-022-00723-z.
- Thyssen JP, Halling AS, Schmid-Grendelmeier P, et al. Comorbidities of atopic dermatitis-what does the evidence say? J Allergy Clin Immunol. 2023;151(5):1155–1162. doi:10.1016/j.jaci.2022.12.002.
- Howe W, Paller AS, Butala S, et al. Treatment of atopic dermatitis (eczema). UpToDate Waltham. 2022;MA2021 [updated 2022 Oct 18]. Available from: https://www.uptodate.com/contents/treatment-of-atopic-dermatitis-eczema/print?search=atopic
- Blauvelt A, Gooderham M, Bhatia N, et al. Tralokinumab efficacy and safety, with or without topical corticosteroids, in North American adults with moderate-to-severe atopic dermatitis: a subanalysis of phase 3 trials ECZTRA 1, 2, and 3. Dermatol Ther. 2022;12(11):2499–2516. doi:10.1007/s13555-022-00805-y.
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413. doi:10.1001/jamadermatol.2022.0029.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi:10.1111/bjd.19574.
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82(2):377–388. doi:10.1016/j.jaad.2019.07.074.
- Wollenberg A, Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–141. doi:10.1016/j.jaci.2018.05.029.
- Silverberg J, Thyssen J, Fahrbach K, et al. Comparative efficacy and safety of systemic therapies used in moderate-to-severe atopic dermatitis: a systematic literature review and network meta-analysis. J Eur Acad Dermatol Venereol. 2021;35(9):1797–1810. doi:10.1111/jdv.17351.
- Kwatra SG, Lio P, Weidinger S, et al. Patient preferences for atopic dermatitis treatments: a discrete choice experiment. J Dermatolog Treat. 2023;34(1):2222201. doi:10.1080/09546634.2023.2222201.
- Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–282. doi:10.1016/S0140-6736(22)01199-0.
- Thomas C, Raibouaa A, Wollenberg A, et al. Patient preferences for atopic dermatitis medications in the UK, France and Spain: a discrete choice experiment. BMJ Open. 2022;12(8):e058799. doi:10.1136/bmjopen-2021-058799.
- Okubo Y, Ho K-A, Fifer S, et al. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatolog Treat. 2020;31(8):821–830. doi:10.1080/09546634.2019.1623860.
- Myers K, Silverberg JI, Parasuraman S, et al. Treatment preferences among patients with mild-to-moderate atopic dermatitis. J Dermatolog Treat. 2023;34(1):2215356. doi:10.1080/09546634.2023.2215356.
- Ameen M, Alhusayen R, Brandi H, et al. Patient preferences in the treatment of moderate-to-severe atopic dermatitis. Acta Derm Venereol. 2024;104:adv24339. doi:10.2340/actadv.v104.24339.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi:10.1056/NEJMoa2019380.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223.
- Silverberg JI, Adam DN, Zirwas M, et al. Tralokinumab plus topical corticosteroids as needed provides progressive and sustained efficacy in adults with moderate-to-severe atopic dermatitis over a 32-week period: an ECZTRA 3 post hoc analysis. Am J Clin Dermatol. 2022;23(4):547–559. doi:10.1007/s40257-022-00702-2.
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi:10.1056/NEJMoa1610020.
- Blauvelt A, Thyssen JP, Guttman-Yassky E, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. 2023;188(6):740–748. doi:10.1093/bjd/ljad022.
- Steven R, Feldman AG, Gauthier-Loiselle M, et al. Understanding patient experience and factors influencing patient preference in the treatment of moderate-to-severe atopic dermatitis through in-depth qualitative patient interviews. Revolutionizing Atopic Dermatitis (RAD) Virtual Conference; 2023 Apr 29–May 1; Washington, DC.
- ChoiceMetrics. Ngene 1.1.1 user manual & reference guide. Sydney (Australia): ChoiceMetrics; 2012.
- Johnson FR, Yang JC, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22(2):157–160. doi:10.1016/j.jval.2018.07.876.
- Gonzalez JM. A guide to measuring and interpreting attribute importance. Patient. 2019;12(3):287–295. doi:10.1007/s40271-019-00360-3.
- Boeri M, Sutphin J, Hauber B, et al. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatolog Treat. 2022;33(3):1449–1458. doi:10.1080/09546634.2020.1832185.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi:10.1016/j.jid.2018.08.028.