Abstract
Purpose
Tildrakizumab is a selective inhibitor of IL-23 approved for the treatment of moderate-to-severe plaque psoriasis in two dosages. We conducted a 16-week multicenter retrospective study to compare the effectiveness and safety of tildrakizumab 200 mg versus tildrakizumab 100 mg in patients with a high disease burden or high body weight.
Materials and methods
Our retrospective study included 134 patients treated with tildrakizumab 200 mg and 364 patients treated with tildrakizumab 100 mg from 28 Italian Dermatology Units affected by moderate-to-severe plaque psoriasis. The patients had a body weight above 90 kg or a high disease burden (Psoriasis Area and Severity Index [PASI] ≥ 16 or the involvement of difficult-to-treat areas). We evaluated the effectiveness of tildrakizumab at the week-16 visit in terms of PASI90, PASI100 and absolute PASI ≤ 2.
Results
After 16 weeks of treatment with tildrakizumab 200 mg, PASI90 was reached by 57.5% of patients and PASI100 by 39.6% of patients. At the same time point, 34.3% and 24.2% of patients treated with tildrakizumab 100 mg achieved PASI90 and PASI100, respectively.
Conclusions
Our data suggest that tildrakizumab 200 mg has better effectiveness than tildrakizumab 100 mg in patients with a body weight ≥ 90 kg and a high disease burden.
Introduction
Psoriasis is a chronic, immune-mediated skin disease that occurs in 2–3% of the general population worldwide (Citation1).
The development of biological drugs has completely changed the treatment of this disease. Among the most recent therapies, an important role is played by monoclonal antibodies that act against pivotal pro-inflammatory cytokines in the pathogenesis of psoriasis, including Interleukin (IL)-23 and IL-17. In particular, IL-23 inhibitors include risankizumab, guselkumab and tildrakizumab (Citation2,Citation3).
Tildrakizumab is a humanized IgG1k monoclonal antibody that selectively binds to the p19 subunit of IL-23 and inhibits its interaction with its receptor. It has been approved for the treatment of moderate-to-severe plaque psoriasis after being evaluated in two phase-III randomized clinical trials (Citation4). Tildrakizumab can be administered in two dosages, 100 mg and 200 mg, at weeks 0, 4, and then every 12 weeks (Citation5). Analyzing the results of the phase-III clinical trials reSURFACE 1 and reSURFACE 2, tildrakizumab 200 mg showed slightly higher efficacy than tildrakizumab 100 mg, but the difference was not statistically significant (Citation4). However, according to a pooled analysis of the reSURFACE studies, tildrakizumab 200 mg demonstrated a higher efficacy compared with tildrakizumab 100 mg in patients with body weight > 120 kg (Citation6).
We conducted a 16-week multicenter retrospective study to compare the effectiveness and safety of tildrakizumab 200 mg versus tildrakizumab 100 mg in patients with moderate-to-severe plaque psoriasis with a high disease burden or high body weight.
Materials and methods
We enrolled 134 patients from 28 Italian Dermatology Units, all affected by moderate-to-severe plaque psoriasis and treated with tildrakizumab 200 mg from September 2023 to February 2024. All patients had a body weight above 90 kg or a high disease burden, including those with a Psoriasis Area and Severity Index (PASI) ≥ 16 or the involvement of difficult-to-treat areas (scalp, palms/soles, nails, and genitalia) (Citation7). This group was compared with 364 patients from the same Dermatology Units, with comparable characteristics at baseline (high disease burden or body weight ≥ 90 kg) that were treated with tildrakizumab 100 mg before tildrakizumab 200 mg became reimbursed in Italy. Patients’ eligibility for tildrakizumab treatment was assessed according to the Italian Adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis (Citation8). All patients were followed for at least 16 weeks and received tildrakizumab 100 or 200 mg according to the Summary of Product Characteristics (Citation5).
The effectiveness of tildrakizumab was evaluated at the week-16 visit in terms of PASI 90 (reduction of at least 90% of PASI compared with baseline), PASI 100 (complete skin clearance) and absolute PASI ≤ 2. Moreover, we compared the effectiveness of tildrakizumab 200 and 100 mg in different patient subgroups. In particular, we compared those with body weight ≥ 90 kg, those with PASI at baseline ≥ 16 and patients with the involvement of at least one difficult-to-treat area. We also recorded the occurrence of any adverse event (AE) at week 16. Categorical variables were reported using absolute numbers and percentages, while continuous data were presented as mean and standard deviation (SD). To assess the statistical differences between tildrakizumab 100 and 200 mg, we used the Chi-squared test and Student’s t-test, as appropriate. A p-value of 0.05 or less was considered statistically significant.
Results
Among the 134 patients treated with tildrakizumab 200 mg, the mean age was 52.90 (SD 13.38), while 88 of them (65.7%) were males. These characteristics were similarly distributed among those treated with tildrakizumab 100 mg (364 patients), with a mean age of 54.38 (15.19) and 242 (66.5%) males. The mean PASI was comparable between the two groups, as it was 14.15 (8.60) in the tildrakizumab 200 mg group versus 14.43 (5.83) in the tildrakizumab 100 mg group. Patients treated with tildrakizumab 200 mg had a higher body weight (91.09 kg [18.87]) compared with those receiving tildrakizumab 100 mg (79.95 kg [17.09]) and were more likely to have the involvement of difficult-to-treat areas (73.9% versus 51.7%). Additional characteristics of both groups at baseline are available in .
Table 1. Demographic characteristics at baseline of patients treated with tildrakizumab 100 and 200 mg.
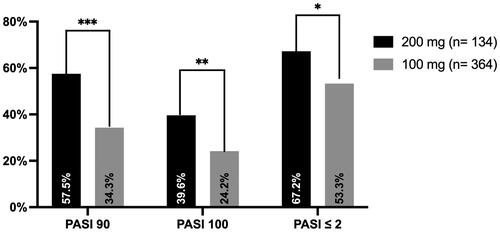
After 16 weeks of treatment (), PASI 90 was reached by 57.5% of patients treated with tildrakizumab 200 mg compared with 34.3% of those receiving tildrakizumab 100 mg (p < 0.001). Complete skin clearance was observed in 39.6% of patients treated with tildrakizumab 200 mg, compared with 24.2% of those in the tildrakizumab 100 mg group (p = 0.001). Regarding absolute PASI, better performances were observed in the tildrakizumab 200 mg group (67.2% vs 53.3%, p = 0.006).
Figure 1. Percentage of patients who achieve PASI 90, PASI 100 and PASI ≤ 2 after 16 weeks of treatment with tildrakizumab 100 and 200 mg.
*p < 0.05; **p < 0.01; ***p < 0.001.
PASI: Psoriasis Area and Severity Index.
Ninety-nine patients (49.5%) treated with tildrakizumab 200 mg had the involvement of difficult-to-treat areas, as compared with 188 (27.5%) in the tildrakizumab 100 mg group. At week 16, those receiving tildrakizumab 200 mg performed better in terms of PASI 90, PASI 100, and PASI ≤ 2 (57.8% vs. 23.2%, 28.9% vs. 12.2%, 60% vs. 34.2%, respectively), with a p-value < 0.05 for all endpoints ().
Figure 2. Percentage of patients achieving PASI 90, PASI 100, and PASI ≤ 2 according to difficult-to-treat areas, body weight ≥ 90 kg, and PASI ≥ 16.
PASI: Psoriasis Area and Severity Index; ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
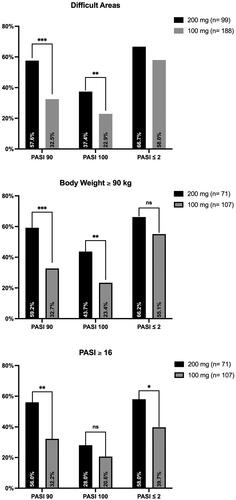
At baseline, a body weight of 90 kg or more was recorded in 71 patients receiving tildrakizumab 200 mg versus 107 treated with tildrakizumab 100 mg. Those in the 200 mg group achieved better PASI 90 and PASI 100 responses (59.2% vs. 32.7%, 43.7% vs. 23.4%, respectively), with a p-value < 0.01 for both endpoints (). Regarding patients with severe psoriasis (PASI ≥ 16), PASI 90 was achieved by 56% of those treated with 200 mg versus 32.2% receiving tildrakizumab 100 mg (p = 0.003), while PASI ≤ 2 was recorded in 58% versus 39.7%, respectively (p = 0.025) ().
No severe AEs or AEs leading to discontinuation were observed during the 16-week follow-up for both groups.
Discussion
Tildrakizumab was evaluated in the phase-3 clinical trials reSURFACE1 and reSURFACE2 across two different dosages (100 and 200 mg), showing comparable efficacy and safety between them. However, post-hoc analyses have demonstrated a superior efficacy of tildrakizumab 200 mg in selected subpopulations. According to a recent study from Dapavo et al. (Citation9), tildrakizumab 200 mg could be the ideal dosage for patients with a high burden of disease (including those with involvement of difficult-to-treat areas, previous exposure to biologics and high baseline PASI) and high body weight.
In our study, at week 16, tildrakizumab 200 showed higher rates of clinical responses compared with the reSURFACE trials, in which PASI 90 was achieved by 35% and PASI 100 by 14% of all patients at week 12 (Citation4). Despite our study being focused on patients with high disease burden, we observed higher effectiveness of tildrakizumab 200 mg compared with clinical trials. Pooled analyses from the reSURFACE studies showed better responses in terms of PASI < 3 in patients with body weight ≥ 90 kg treated with tildrakizumab 200 mg compared with tildrakizumab 100 mg after 28 weeks (Citation5). Despite a shorter follow-up period in our study, we observed similar effectiveness in patients with a body weight of 90 kg or more receiving tildrakizumab 200 mg, as PASI ≤ 2 was reached by 66.2% while PASI < 3 was achieved by 66.3% in the pooled analyses (Citation6).
Tildrakizumab has shown effectiveness in multiple real-world studies, but the role of the 200 mg dosage has yet to be fully explored (Citation10–12). Becher et al. included 33 patients over 90 kg of weight treated with tildrakizumab 200 mg in a real-world study observing higher clinical effectiveness (Citation13).
Regarding the safety of tildrakizumab, no significant findings have emerged in real-world clinical practice to date for both dosages (Citation4, Citation10).
Our study has a few limitations due to its retrospective design. First, patients from the two treatment groups were enrolled during different time frames because of the later approval of tildrakizumab 200 mg in Italy. Second, due to the multicentric nature of the study, patients were evaluated by different clinicians, resulting in heterogeneous clinical assessments. Finally, the follow-up period is limited, and it does not allow us to properly evaluate the safety profile of tildrakizumab 200 mg. However, to date, our study is the first real-world experience on using this dosage of tildrakizumab in different patients’ phenotypes.
In conclusion, in our experience, tildrakizumab 200 mg showed better effectiveness than tildrakizumab 100 mg in patients with a body weight ≥ 90 kg and a high disease burden. In particular, tildrakizumab 200 mg performed better after 16 weeks in those with severe plaque psoriasis and involvement of difficult-to-treat areas. Longer and larger studies are needed to investigate further the role of tildrakizumab 200 mg in different subpopulations of patients with plaque psoriasis.
Compliance with ethics guidelines
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received upadacitinib as in good clinical practice, in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Acknowledgments
This work was partially supported by ‘Ricerca Corrente’ funding from Italian Ministry of Health to IRCCS Humanitas Research Hospital.
Disclosure statement
L. Gargiulo has been a consultant and/or speaker for Almirall, UCB and Pfizer. L. Ibba has been a consultant for Almirall. P. Malagoli has been a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma, and Almirall. A. Balato has received honoraria for participation in advisory boards, meetings, or as speaker for AbbVie, Celgene, Janssen-Cilag, Eli Lilly, Novartis Pharma, Pfizer, Sanofi-Genzyme, and UCB Pharma. F. Bardazzi has been a consultant adviser and clinical study investigator for Eli Lilly, Abbvie, Novartis, Leo Pharma, Sandoz, Bristol Myers, Abiogen-Pharma, Celgene and Janssen. M. Burlando has acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, UCB Pharma. C. G. Carrera has served as a board participant or speaker for Abbvie, Lilly, Janssen, Novartis, Celgene, Almirall, and Leopharma. P. Dapavo has been a speaker for Novartis, Abbvie, Sanofi, UCB, Janssen, Lilly, and LeoPharma. F. M. Gaiani acted as a speaker or consultant for Novartis, Abbvie, Eli Lilly, Celgene, LeoPharma, and Almirall. P. Gisondi has been a consultant and/or speaker for Abbvie, Almirall, Amgen, Janssen, Leo-Pharma, Eli-Lilly, Novartis, Pierre Febre, Sandoz, Sanofi and UCB. C. Guarneri has been a scientific consultant/speaker/clinical study investigator for Abbvie, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Almirall, LEO Pharma. C. Lasagni declares a conflict of interest with Abbvie, Novartis, Lilly and Almirall. F. Loconsole served on advisory boards and/or received honoraria for lectures from Abbvie, Janssen-Cilag, Novartis, Lilly, Sanofi. A. V. Marzano reports consultancy/advisory boards disease-relevant honoraria from AbbVie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi and UCB. M. Megna acted as a speaker or consultant for Abbvie, Eli Lilly, Janssen, Leo-Pharma, and Novartis. M. L. Musumeci has previously served as advisory board member and consultant, and has received speaker’s honoraria and fees for her participation to clinical trials for Abbvie, Almirall, Biogen, Eli-Lilly, Janssen Cilag, Leo Pharma, and Novartis. D. Orsini has been a speaker and/or consultant for Abbvie, LeoPharma, uCB, Bristol-Meyer-Squibb and Boehringer-Ingelheim. S. Ribero has served as advisory board member and/or consultant and has received fees and speaker’s honoraria or has participated for clinical studies for AbbVie, Almirall, Leo Pharma, Eli Lilly, Novartis, Pfizer and Sanofi Genzyme. D. Strippoli has been a consultant and/or speaker for Abbvie, Celgene, Janssen, Eli Lilly, Novartis, Pfizer. E. Trovato is involved in an intermittent project focused on consulting and/or advisory relationships or/and travel-congress support with Eli-Lilly, Novartis, Janssen-Cilag, Abbvie and Almirall. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim. M. Venturini served as a speaker or advisory board member for Abbvie, Almirall, Amgen, Eli-Lilly, Galderma, Leo Pharma, Novartis, Pierre Fabre, and UCB Pharma. L. Zichichi has received grants for scientific contributions from AbbVie, Almirall, Amgen, Lilly and Novartis. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim. R. Cascio Ingurgio, F. Amoruso, P. Brianti, G. Brunasso, A. E. Cagni, M. Caproni, A. Carugno, F. Caudullo, A. Cuccia, E. V. Di Brizzi, V. Dini, G. Licata, S. R. Mercuri, V. Ruffo Di Calabria, F. Satolli, M. Travaglini have nothing to declare.
Data availability statement
All the patients’ data and information supporting the findings of the study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315. Apr 3 doi:10.1016/S0140-6736(20)32549-6.PMID: 33812489.
- Sbidian E, Chaimani A, Guelimi R, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2023;7(7):CD011535. Published 2023 Jul 12. doi:10.1002/14651858.CD011535.pub6.
- Valenti M, Narcisi A, Pavia G, et al. What can IBD specialists learn from IL-23 trials in dermatology? J Crohns Colitis. 2022;16(Supplement_2):ii20–ii29. doi:10.1093/ecco-jcc/jjac023.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials [published correction appears in lancet. 2017 jul 15;390(10091):230]. Lancet. 2017;390(10091):276–288. doi:10.1016/S0140-6736(17)31279-5.
- European Medicines Agency. Ilumetri (tildrakizumab): sumìmary of product characteristics. 2018 [cited 2024 February 14]. https://www.ema.europa.eu/en/medicines/human/EPAR/ilumetri.
- Thaçi D, Gerdes S, Du Jardin KG, et al. Efficacy of tildrakizumab Across different body weights in moderate-to-severe psoriasis Over 5 years: pooled analyses from the reSURFACE pivotal studies. Dermatol Ther (Heidelb). 2022;12(10):2325–2341. Oct Epub 2022 Sep 13. PMID: 36098877; PMCID: PMC9515266. doi:10.1007/s13555-022-00793-z.
- Ibba L, Gargiulo L, Alfano A, et al. Anti-IL-23 and anti-IL-17 drugs for the treatment of non-pustular palmoplantar psoriasis: a real-life retrospective study. J Dermatolog Treat. 2023;34(1):2199108. doi:10.1080/09546634.2023.2199108.
- Gisondi P, Fargnoli MC, Amerio P, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol. 2022;157(Suppl. 1 to No. 1):1–78. doi:10.23736/S2784-8671.21.07132-2.
- Dapavo P, Burlando M, Guarneri C, et al. Tildrakizumab: the value of a personalized and flexible approach for treating moderate-to-severe plaque psoriasis in patients with high body weight or high disease burden. Expert Opin Biol Ther. 2024;24(3):133–138. Published online March 5 doi:10.1080/14712598.2024.2325547.
- Narcisi A, Valenti M, Gargiulo L, et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicentre retrospective study-IL PSO (italian landscape psoriasis). J Eur Acad Dermatol Venereol. 2023;37(1):93–103. doi:10.1111/jdv.18594.
- Costanzo A, Llamas-Velasco M, Fabbrocini G, et al. Tildrakizumab improves high burden skin symptoms, impaired sleep and quality of life of moderate-to-severe plaque psoriasis patients in conditions close to clinical practice. J Eur Acad Dermatol Venereol. 2023;37(10):2004–2015. doi:10.1111/jdv.19229.
- Berenguer-Ruiz S, Aparicio-Domínguez M, Herranz-Pinto P, et al. Effectiveness and safety of tildrakizumab for the treatment of psoriasis in real-world settings at 24 weeks: a retrospective, observational, multicentre study by the spanish psoriasis group. J Eur Acad Dermatol Venereol. 2023;37(12):2517–2525. doi:10.1111/jdv.19468.
- Becher G, Conner S, Ingram JA, et al. A retrospective real-world study of the effectiveness and tolerability of tildrakizumab in UK adults with moderate-to-severe chronic plaque psoriasis. Dermatol Ther (Heidelb). 2022;12(10):2343–2354. doi:10.1007/s13555-022-00800-3.

