Abstract
An efficient conversion of ortho, meta and para fluoro- and trifluoromethyl-substituted benzoic acids to the corresponding benzamides in fermentations of the soil bacterium Streptomyces sp. JCM9888 is described. We also report the efficient reduction of the same class of substrates to the corresponding benzyl alcohols with the fungi Cunninghamella elegans. These biotransformations were surprisingly efficient and may have value as disruptive technologies in process chemistry.
1. Introduction
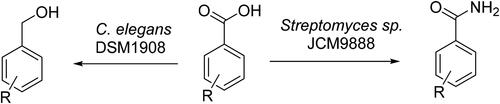
Given the requirement for sustainable processes, biotransformations are becoming an increasing focus of attention in the development of disruptive technologies for process chemistry (Sheldon and Pereira Citation2017). There are a myriad of reactions represented in biotransformations, many involving the oxidation and reduction of functional groups as these represent among the most desirable transformations in synthetic chemistry (Klatte and Wendisch Citation2014). Also, amide formations from carboxylic acids are claimed to account for around one fifth of all chemical transformations conducted in industry, although there are remarkably few biotransformation protocols that make amides from carboxylic acids (Wood et al. Citation2017; Dorr and Fuerst Citation2018). It was in this context that we decided to report some recent unexpected observations in the biotransformations of selectively fluorinated benzoic and related acids. There are reports over the years addressing the reduction of benzoic acids using various fungal strains such as Dichomitus albidofuscus (white rot fungi) (Zhuk et al. Citation2021), Nocardia asteroids (Kato et al. Citation1988), Desulfomicrobium escambiense (Genthner et al. Citation1997), Desulfovibrio vulgaris PY1 (Bock et al. Citation2000) and Pyrococcus furiosus (van den Ban et al. Citation1999). In this paper, we report a similar reductive transformation with the fungus Cunninghamella elegans. This fungus is more widely known as a model of mammalian P-450 oxidative metabolism and a number of laboratories have explored its ability to oxidatively degrade organofluorine compounds (Asha and Vidyavathi Citation2009; Amadio and Murphy Citation2010). Our original objective was to challenge the fungus with ortho, meta and para fluoro- and trifluoromethyl-substituted benzoic acids, to explore halide metabolism. In the event, these compounds proved stable to dehalogenation, but instead they underwent very efficient carboxylate reduction to their corresponding benzyl alcohols. Interestingly too, when we incubated the benzoic acids with Streptomyces soil bacteria, we observed that Streptomyces sp. JCM9888 (Isono et al. Citation1984) was able to convert the benzoic acids to the corresponding benzamides with high efficiency. This study was extended to fluorinated cinnamic and phenylacetic acids and their respective amides were observed. Although a very common chemical transformation, this is a rare biotransformation that may prove of some value as a disruptive technology.
2. Materials and methods
2.1. Chemicals
The benzoic acid substrates were of analytical grade purchased from Sigma Aldrich (Gillingham, UK, St. Louis, MO, and Darmstadt, Germany), Alfa Aesar (Shanghai, China) and Fluorochem (Hadfield, UK).
2.2. Microbes
Cunninghamella elegans DSM1908 was obtained from the German Collection of Microorganisms and Cell Cultures GmbH (Braunschweig, Germany). Streptomyces sp. JCM9888 used for this study was obtained from the Japan Collection of Microorganisms (Tsukuba, Japan).
New plates of Cunninghamella elegans were cultivated according to the procedure described by Amadio et al. (2010) and Khan and Murphy (2021). Briefly, the fungal mycelia were first grown on Sabouraud dextrose agar (SDA) plates (for five days at 28 °C) from an inoculum prepared by homogenizing the mycelium of the fungus (DSM1908) in 100 mL of sterile saline solution (0.8% w/v) and stored at 4 °C on maturation. Liquid cultures of the fungi were grown in Erlenmeyer shake flasks (250 mL) containing sterile Sabouraud dextrose broth (50 mL) inoculated with a uniform square cut of mature SDA gels of C. elegans and incubated for 72 h at 28 °C and 150 rpm agitation. The fungus was then used for biotransformation experiments by adding the benzoic acid substrates (7–8 mg) dissolved in dimethylformamide (DMF) (50 µL) and then further incubated for 72 h at 28 °C at 150 rpm. All incubations were carried out in triplicate.
The fungal biomass was separated from the supernatant, and the supernatant extracted with ethyl acetate (3 × 50 mL) and then dichloromethane (3 × 50 mL). The organic extracts were combined and evaporated for NMR analysis.
Analysis of the resulting extracts was carried out by 1H NMR and 19F{1H} NMR on 400 MHz and 500 MHz Bruker Avance spectrometers (Billerica, MA). Metabolites were further isolated via column chromatography using Merck Geduran silica gel (Kenilworth, NJ; 250–400 mesh) eluting with dichloromethane and with added ethyl acetate (10–20%) to add polarity.
Spores of Streptomyces sp. JCM9888 were grown following published protocols (Zhao et al. Citation2014) on ISP4 agar plates (per litre: 10 g soluble starch, 1 g K2HPO4, 1 g MgSO4·7H2O, 1 g NaCl, 1 g (NH4)2SO4, 1 g CaCO3, 20 g agar) incubated at 30 °C for five days. Spores of S. sp. JCM9888 were added to tryptic soya broth (TSB) media (50 mL made from per litre: 30 g trypticase soy broth, 3 g yeast extract, 13 g NaCl, 0.34 g KCl, 4 g MgCl2·6H2O, 3.45 g MgSO4·7H2O, 0.25 g NH4Cl, 0.14 g CaCl2) and incubated for two days at 28 °C on a shaker (150 rpm). An aliquot (1 mL) of this preculture was used to inoculate 50 mL of fermentation broth (per litre: 5 g cotton seed media, 5 g yeast extract, 10 g soluble starch, 10 mM MgSO4·7H2O, 0.001 g CoCl2, FeSO4·7H2O) (Takahashi and Beppu Citation1982). The culture was shaken (150 rpm) in Erlenmeyer shake flasks (250 mL) for 48 h. On day 3, the benzoic acids (7–8 mg) were added to the fermentation broth and incubated for six days. The cultures were harvested and centrifuged (6000 rpm, 4025 × g, 12 min), and the supernatant was extracted with ethyl acetate (2 × 50 mL). Products were purified by column chromatography.
3. Results and discussion
In the first instance, the selectively fluorinated ortho, meta and para, fluorobenzoic acids 1b–d, the structures of which are shown in , were incubated with C. elegans as described in Section 2.
Figure 1. Benzoic 1a–e and 2a–c, phenylacetic 3a–b and cinnamic acids 4a–b used as substrates for biotransformations.

After three days, the product extracts were analysed by 19F NMR and in all cases there had been an efficient conversion to new fluorinated products (). Purification and analysis indicated that these new products were exclusively the corresponding benzyl alcohols 5a–d, respectively. The identity of the benzyl alcohols was verified by 1H- and 19F NMR by comparison with reference compounds. There was no evidence of any other products being generated by 19F NMR, so both conversions and selectivity were judged to be high, although conversion of the ortho fluoro benzoic acid 1d to benzyl alcohol 5d was the most sluggish. Similarly, the three trifluoromethyl benzoic acid isomers 2a–c were incubated in cultures of C. elegans and again these underwent selective and efficient bio-conversions to benzyl alcohols 6a–c, certainly for the meta and para benzoic acids. Again, the identity of the corresponding benzyl alcohols was determined by 1H- and 19F NMR comparison with reference benzyl alcohols after purification by chromatography. All transformations were carried out in triplicate and we note particularly efficient transformations for the meta and para substituted substrates. For ortho-fluoro benzoic acid 1d, the transformation was noticeably slower and there was no conversion at all observed for ortho-trifluoromethylbenzoic acid 2c, perhaps consistent with the steric impact of the ortho substituent close to the carbonyl on the reducing enzyme. There are some reports in the literature (e.g. Kato et al. Citation1988; Genthner et al. Citation1997; van den Ban et al. Citation1999; Bock et al. Citation2000; Zhuk et al. Citation2021) where fungi have been shown to have the ability to reduce carboxylic acids, and particularly benzoic acids in this way; however, this study significantly extends the range of fluorinated benzoic acids and used the model organism C. elegans.
Figure 2. Bioconversions of benzoic acids 1–2 to benzyl alcohols 5–6 with Cunninghamella elegans. Conversions (%) are indicated in each case for three day incubations as averages of at least duplicate experiments.

The monofluoro and trifluoromethyl benzoic acids 1b–d and 2a–c () were then explored in incubations with two Streptomyces bacterial strains, Streptomyces calvus and Streptomyces sp. JCM9888. These particular strains were selected as they are of current interest in our laboratory as halogenated sulfamoyladenosine antibiotic producers (Zhao et al. Citation2014; Wojnowska et al. Citation2023). Incubations with S. calvus did not result in any obvious biotransformation of the fluorinated benzoic acids to amides; however, incubations with Streptomyces sp. JCM9888 gave highly efficient conversions to new products (). This was immediately obvious by 19F {1H} NMR analysis of the organic extracts after work-up. In all cases, these products proved to be the corresponding benzamides 7b–d and 8a–c. Their identities were confirmed by preparing reference amides from the carboxylic acids via their acid chlorides, by reaction with oxalyl chloride, and then ammonium chloride treatment (see SI).
Figure 3. Biotransformations of aromatic carboxylic acids 1a–e, 2a–c, 3a–b, 4a–b to amides 7a–e, 8a–c, 9a–b, 10a–b with Streptomyces sp. JCM9888. Conversions are indicated in each case for six day incubations of the various substrates.

The biotransformation of a carboxylic acid to a primary amide is a rare transformation (Kergomard and Renard Citation1986; Dorr and Fuerst Citation2018) and was somewhat unexpected, certainly given the high conversions observed here. Benzoic acid itself 1a, 2,3-difluorobenzoic acid 1e, 4′- and 2′-fluorophenylacetic acid 3a–b and cinnamic acid 4a–b was also explored as substrates and these too were bio-converted to benzamide 7a, 7e with good efficiency and amide 9a–b, 10a–b were also observed but at a lower conversion.
4. Conclusions
We report here the efficient conversions of selectively fluorinated benzoic acids to their corresponding benzylalcohols with the fungi, Cunninghamella elegans. Several fungi have been reported to carry out reductive biotransformations on benzoic acids; however, C. elegans is a model organism known for oxidative metabolism and therefore this observation seemed counter-intuitive and adds to an appreciation of its metabolic versatility. Incubation of the halogenated benzoic acids with the bacterium Streptomyces sp. JCM9888 resulted in efficient conversions to the corresponding benzamides. Biotransformations of carboxylic acids to primary amides is rare, despite this being a common chemical transformation, and thus these protocols may be offer some advantage in industrial biotransformation technology.
Author contributions
Oluwayinka O. Oke designed and performed the microbiology and synthesis experiments and helped develop the manuscript. Yawen Chen assisted with the microbiology aspects of the project. Chukwuemeka Isanbor and Olayinka T. Asekun guided the research and assisted in funding support. David O’Hagan led the project, guided the research and drafted the manuscript.
Supplemental Material
Download MS Word (135 KB)Acknowledgements
We are grateful to the Commonwealth Scholarship Commission and also the Royal Society of Chemistry for their support. We are also extremely grateful to Prof Cormac D. Murphy of University College Dublin for a strain of C. elegans.
Disclosure statement
The authors report there are no conflicts of interest.
Additional information
Funding
References
- Amadio J, Murphy CD. 2010. Biotransformation of fluorobiphenyl by Cunninghamella elegans. Appl Microbiol Biotechnol. 86(1):345–351. doi: 10.1007/s00253-009-2346-4.
- Asha S, Vidyavathi M. 2009. Cunninghamella – a microbial model for drug metabolism studies – a review. Biotechnol Adv. 27(1):16–29. doi: 10.1016/j.biotechadv.2008.07.005.
- Bock M, Kneifel H, Schoberth SM, Sahm H. 2000. Reduction of halogenated derivatives of benzoic acid to the corresponding alcohols by Desulfovibrio vulgaris PY1. Acta Biotechnol. 20(3–4):189–201. doi: 10.1002/abio.370200303.
- Dorr BM, Fuerst DE. 2018. Enzymatic amidation for industrial applications. Curr Opin Chem Biol. 43:127–133. doi: 10.1016/j.cbpa.2018.01.008.
- Genthner BRS, Townsend GT, Blattmann BO. 1997. Reduction of 3-chlorobenzoate, 3-bromobenzoate and benzoate to corresponding alcohols by Desulfomicrobium escambiense isolated from a 3-chlorobenzoate dechlorinating coculture. Appl Environ Microbiol. 63(12):4698–4703. doi: 10.1128/aem.63.12.4698-4703.1997.
- Isono K, Uramoto M, Kusakabe H, Miyata N, Koyama T, Ubukata M, Sethi SK, McCloskey JA. 1984. Ascamycin and dealanylascamycin, nucleoside antibiotics from Streptomyces sp. J Antibiot. 37(6):670–672. doi: 10.7164/antibiotics.37.670.
- Kato N, Konishi H, Uda K, Shimao M, Sakazawa C. 1988. Microbial reduction of benzoate to benzyl alcohol. Agric Biol Chem. 52:1885–1886.
- Kergomard A, Renard MF. 1986. Action of two strains of Streptomyces on aromatic substrates. Agric Biol Chem. 50(11):2913–2914. doi: 10.1080/00021369.1986.10867848.
- Klatte S, Wendisch VF. 2014. Redox self-sufficient whole cell biotransformation for amination of alcohols. Bioorg Med Chem. 22(20):5578–5585. doi: 10.1016/j.bmc.2014.05.012.
- Khan MF, Murphy CD. 2021. Bacterial degradation of the anti-depressant drug fluoxetine produces trifluoroacetic acid and fluoride ion. Appl Microbiol Biotechnol. 105(24): 9359–9369 doi: 10.1007/s00253-021-11675-3
- Sheldon RA, Pereira PC. 2017. Biocatalysis engineering; the big picture. Chem Soc Rev. 46(10):2678–2691. doi: 10.1039/c6cs00854b.
- Takahashi E, Beppu T. 1982. A new nucleoside antibiotic. J. Antibiotic 35(8): 939–947. doi: 10.7164/antibiotics.35.939
- van den Ban ECD, Willemen HM, Wassink H, Laane C, Haaker H. 1999. Bioreduction of carboxylic acids by Pyrococcus furiosus in batch cultures. Enzyme Microb Technol. 25(3–5):251–257. doi: 10.1016/S0141-0229(99)00036-8.
- Wojnowska M, Feng X, Chen Y, Deng H, O'Hagan D. 2023. Identification of genes essential for fluorination and sulfamylation within the nucleocidin gene clusters of Streptomyces calvus and Streptomyces virens. Chembiochem. 24(5):e2022006. doi: 10.1002/cbic.202200684.
- Wood JL, Weise NJ, Frampton JD, Dunstan MS, Hollas MA, Derrington SR, Lloyd RC, Quaglia D, Parmeggiani F, Leys D, et al. 2017. Adenylation activity of carboxylic acid reductases enables the synthesis of amides. Angew Chem Int Ed Engl. 56(46):14498–14501. doi: 10.1002/anie.201707918.
- Zhao CH, Qi JZ, Tao WX, He L, Xu W, Chan J, Deng ZX. 2014. Characterization of biosynthetic genes of ascamycin/dealanylascamycin featuring a 5′-O-sulfonamide moiety in Streptomyces sp. JCM9888. PLOS One. 9(12):e114722. doi: 10.1371/journal.pone.0114722.
- Zhuk TS, Skorobohatko OS, Albuquerque W, Zorn H. 2021. Scope and limitations of biocatalytic reduction with white rot fungi. Bioorg Chem. 108:104651. doi: 10.1016/j.bioorg.2021.104651.