?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chrysotile was formerly used in the manufacture of casting ring liner (CRL) and periodontal dressing powder (PDP). The purpose of this study was to describe the potential for airborne asbestos exposure among dental professionals who may have used these products and to assess their risk of asbestos-related disease (ARD). Task-specific exposure data associated with CRL and PDP were identified and compared to regulatory standards for asbestos and health-based benchmarks. Personal airborne fiber concentrations ranged from 0.008–3.5 f/cc by PCM (duration: 3–420 minutes) for CRL (tearing, placement), and from <0.0044–<0.297 f/cc by PCM (duration: 5–28 minutes) for PDP (mixing). Eight-hour time-weighted average (TWA) exposures were calculated using the reported task-based airborne fiber concentrations and associated sampling durations. For CRL tasks, the upper-bound calculated 8-hour TWA of 0.022 f/cc (tearing, placement) did not exceed regulatory standards for asbestos (≥0.1 f/cc). All samples collected during the mixing of PDP resulted in non-measurable fiber concentrations. The greatest estimated cumulative asbestos exposure for dental professionals using CRL (tearing, placement) of 0.33 f/cc-years is well below “best estimate”, published chrysotile no-observed-adverse-effect-levels (NOAEL) for ARD (lung cancer = 89–168 f/cc-years; pleural mesothelioma = 208–415 f/cc-years). As such, the use of asbestos-containing CRL and/or PDP is not expected to pose an increased risk of ARD among dental professionals. This conclusion is consistent with the lack of an increased risk of ARD reported in epidemiological studies of these occupations.
Introduction
In 1950, approximately 157,000 individuals worked in dental offices in the U.S., over half of whom were dentists (51%; n = 80,070), 2% (3,140) were dental hygienists, 35% (54,950) were dental assistants, and 11% (17,270) were “other staff”, including secretaries, receptionists, office managers, etc. (Solomon Citation2012, p. 1031). The number of dentists in the U.S. has doubled since 1950, with even sharper increases in the number of dental hygienists and dental assistants (Solomon Citation2012). To the best of our knowledge, historical employment data for dental laboratory technicians is not readily available.
According to the U.S. Bureau of Labor Statistics (BLS), as of 2018, there were approximately 802,300 dental personnel, including dentists (155,000), dental laboratory technicians (81,500), dental hygienists (219,800), and dental assistants (346,000), in the U.S. (Bureau of Labor Statistics [BLS] Citation2019a, 2019b, 2019c, 2019d). Dentists diagnose and treat medical issues in patients’ teeth, gums, and other parts of the mouth, typically in an office setting (Bureau of Labor Statistics [BLS] Citation2019d). These professionals may also undergo training during dental school to learn how to create dental fixtures like crowns and bridges, and may perform such tasks while practicing as a licensed dentist. Dental laboratory technicians are mainly responsible for constructing, fitting, and/or repairing dental fixtures in laboratories (Bureau of Labor Statistics [BLS] Citation2019a). Dental hygienists largely work in dentists’ offices, where they examine patients for indications of oral disease and provide oral hygiene preventive care (Bureau of Labor Statistics [BLS] Citation2019c). Dental assistants generally provide patient care, take X-rays, maintain records, and perform other administrative tasks in dentists’ offices, such as scheduling appointments (Bureau of Labor Statistics [BLS] Citation2019b).
At various points in time, dental professionals may have experienced a variety of chemical exposures, including potential exposure to alginate powders, nitrous oxide, mercury, asbestos, beryllium, other non-precious metal alloys, silica, methylmethacrylate, and formaldehyde (Woody et al. Citation1977; Setcos et al. Citation1983; Rom et al. Citation1984; Wagner Citation1985; Persson and Brune Citation1989; Palmen Citation2005; Torbica and Krstev Citation2006). Of note, chrysotile was historically used in the manufacture of various dental products, including casting ring liner (CRL; i.e. dental tape), between approximately 1930 and 1987 (Taylor et al. Citation1930; Naylor et al. Citation1987), investment material (i.e. refractory material) from an unspecified year until 1930 (Broomell Citation1908; Souder and Sweeney Citation1930; Taylor et al. Citation1930), and periodontal dressing powder (PDP) between approximately 1923 and 1976 (Dyer Citation1967; Council on Dental Therapeutics [CDT] Citation1976; Cook Citation2008; Baghani and Kadkhodazadeh Citation2013).
Casting ring liner (CRL) and investment material
CRL and investment material are two products typically used by dental professionals during the “lost-wax” casting process. This method dates back to ancient Egypt, and was also used by early Chinese civilizations, as well as the Etruscans, Romans, Aztecs, Mayans, and Phoenicians (Hollenback Citation1962; Morey Citation1991a). The casting process that is used in dentistry, however, was first developed in 1907 by Dr. W. H. Taggart (Taggart Citation1907). The “lost-wax” casting process is used by dental professionals to create crowns, bridges, and other dental fixtures by forming and then filling imprints of wax models or inlays in hardened investment material. The use of a wetted asbestos CRL as part of the “lost-wax” method was used to allow for the proper expansion of the investment material during the casting process to ensure accuracy of the metal casting (Souder and Paffenbarger Citation1942; J. F. Jelenko & Co. Citation1963; Skinner Citation1963). Although the use of asbestos-containing CRL was first described by Taylor et al. in 1930, it became a universally adopted casting technique in 1960 when it was introduced by Dr. Wilmer A. Souder of the U.S. National Bureau of Standards (NBS) (Taylor et al. Citation1930; Hollenback and Rhoads Citation1960; Earnshaw Citation1988).
In 1976, the Council on Dental Therapeutics (CDT) and the Council on Dental Materials and Devices (CDMD) reported that personnel could be exposed to airborne asbestos in dental laboratories where asbestos-containing CRL was stored in large rolls and subsequently used in the casting process. This potential asbestos exposure was due to “the tendency for personnel to carelessly cut sections off these rolls and thus release asbestos into the ambient air” (Council on Dental Therapeutics [CDT] Citation1976, p. 777). Despite these concerns, however, CRL was not removed from the CDT’s “Acceptance Program” at the time. Instead, the CDT and CDMD recommended that wet methods be used when handling and manipulating asbestos-containing CRL in dental laboratories.
Since at least the mid-1970s, ceramic, cellulose, and ceramic-cellulose CRLs, and even “strips of card obtained from a breakfast cereal packet”, have been assessed and utilized as product substitutes for asbestos-containing CRLs, though a consensus has not been formed as to which products produce the most accurate castings (Burnett Citation1976; Priest and Horner Citation1980; Yli-Urpo et al. Citation1982; Brantley et al. Citation1986; Naylor et al. Citation1987; Earnshaw Citation1988; Morey Citation1992b). By 1987, the use of asbestos-containing CRLs was noted to have been “virtual[ly] eliminat[ed]” (Naylor et al. Citation1987, p. 413).
The investment material, which was used to create the casting during the “lost-wax” method, possibly also contained asbestos for an unspecified period of time to aid in heat resistance and to prevent fracturing of the material (Broomell Citation1908). In July 1930, the U.S. NBS approved the American Dental Association’s (ADA) specification for dental inlay casting investment (Souder and Sweeney Citation1930; Taylor et al. Citation1930). According to the specification, the investment material “shall be a powder composed essentially of plaster of Paris and silica, or other material of these types” and “free of foreign material”, excluding coloring material (Taylor et al. Citation1930, p. 2284). Per the ADA specification, the presence of asbestos in an investment material product precluded it from being approved (Broomell Citation1908; Souder and Sweeney Citation1930; Taylor et al. Citation1930). Whether the ADA’s specification that investment material not contain asbestos fibers was based on health concerns or if it was simply thought to negatively affect the nature of the investment material remains unclear, however. Notably, it has been demonstrated that both the composition of the investment material and the allotted amount of setting time affect the total expansion of investment material, which is a key factor in the creation of a precisely-sized dental prosthesis (Asgarzadeh et al. Citation1954).
Periodontal dressing powder (PDP)
PDP, also referred to as asbestos resin dressing and gingivectomy packs, was first used beginning in 1923 following periodontal surgery to treat patients’ wounds (Dyer Citation1967; Cook Citation2008; Baghani and Kadkhodazadeh Citation2013). In Citation1976, the CDT and CDMD determined that the “potential danger to dental personnel in preparation of asbestos-containing periodontal packs [was] of sufficient concern”, such that PDP was no longer considered eligible for the CDT’s Acceptance Program as of this same (1976) year (Council on Dental Therapeutics [CDT] Citation1976, p. 777). This decision to remove asbestos-containing PDP from the Acceptance Program was commended by the National Institute for Occupational Safety and Health (NIOSH) at the time (Infante and Lemen Citation1976). While officials were concerned with potential health risks posed to dental professionals from the use of PDP, the risk of asbestos exposure in patients was not of concern. Indeed, in 1976, the CDT and CDMD noted that “[t]here [was] no apparent health danger from inhalation to patients for whom asbestos-containing periodontal dressings [were] used, since the fibers cannot be appreciably released from the dressings once they [were] mixed and applied. In addition, present information indicates that there [was] little danger to patients should the dressings be accidentally swallowed” (Council on Dental Therapeutics [CDT] Citation1976, p. 778).
The purpose of the present study was to characterize the potential for historical airborne asbestos exposure in dentists and other dental professionals, including dental laboratory technicians, dental hygienists, and dental assistants, who personally used or were bystanders to the use of these products on a regular basis in an occupational setting. Specifically, a literature review was performed in order to estimate potential airborne asbestos exposures of dental professionals during typical use and manipulation of CRL, investment material, and PDP. Eight-hour time-weighted average (TWA) exposure estimates were calculated for each product-specific task for which measurable data were available, and compared to historical and current regulatory standards for asbestos. The upper-bound 8-h TWA for each product-specific task was then used to estimate cumulative asbestos exposures, which were ultimately compared to health-based, chrysotile no-effect levels for asbestos-related disease (ARD), specifically lung cancer and pleural mesothelioma. Although non-asbestos risk factors for lung cancer and pleural mesothelioma have been previously described (Pesch et al. Citation2012; Attanoos et al. Citation2018), the scope of the current risk assessment was limited to potential lung cancer and pleural mesothelioma risk from asbestos exposures among dental professionals.
Methods
Literature search
A comprehensive literature search was performed to identify published studies regarding asbestos in dental products in order to characterize potential asbestos exposures associated with the typical use of these products. A PubMed database search was performed using the following search terms in the specified combinations: (“asbestos” AND “dental”); (“asbestos” AND “dentist”); (“asbestos” AND “casting”); (“asbestos” AND “ring liner”); (“asbestos” AND “periodontal dressing”). If additional studies were identified during review of cited references, but not captured in the original database search, they were also included in the analysis. Unpublished reports received through litigation and their references were also reviewed and those that were found to be relevant to our analysis are included as Supplemental Material. Dental textbooks, as well as dental professional association standards and specifications, were also consulted to determine typical product use. After each relevant study and/or report was identified and reviewed, it was included in one or more of the following categories based on its content: (1) Product Use, (2) Bulk Product Analyses, (3) Animal Inhalation Toxicity Studies, (4) Fiber Release Studies, (5) Exposure Studies, and/or (6) Human Health Studies, which is how each study and/or report is presented herein.
Exposure assessment
Cumulative asbestos exposure estimates (f/cc-years) were derived by first calculating personal 8-h TWA exposures (f/cc) based on the measured airborne fiber concentration data identified in the published and unpublished literature. Any area samples reported in the literature were also used to approximate bystander 8-h TWA exposures in environments where these dental products were typically used. To calculate 8-h TWA concentrations, airborne fiber concentrations and the associated sampling durations for each discrete sample collected and reported in the literature were used. Short-term exposure measurements were converted to 8-h TWA concentrations according to EquationEquation (1)(1)
(1) . It was assumed that there was no other asbestos exposure throughout the day except during the sampling duration.
(1)
(1)
where CTWA=8-h TWA concentration (f/cc); CT=Task-based airborne fiber concentration (f/cc); TD=Task-based exposure duration (min).
An upper-bound 8-h TWA concentration, defined as the greatest 8-h TWA in the available dataset (both reported and calculated values), was then identified for each product-specific task for which measurable data were available for the purpose of calculating a cumulative asbestos exposure estimate. Cumulative asbestos exposures (f/cc-years) were specifically calculated by multiplying the upper-bound 8-h TWA (f/cc), which was calculated from airborne fiber concentration/sampling duration pairs reported in the available literature as described above, by mean duration of employment (years) for each profession (i.e. dentist, dental laboratory technician, dental hygienist, and dental assistant), according to Maguire (Citation1993), assuming a workday duration of 8 h (480 min) and an occupational year of 250 days (EquationEquation (2)(2)
(2) ). Potential asbestos exposures to dental patients were outside the scope of this analysis and were therefore not evaluated.
(2)
(2)
where CE=Cumulative asbestos exposure (f/cc-years); CTWA,MAX=Upper-bound 8-h TWA concentration (f/cc); EF= Exposure frequency in number of years of product use (years); ED=Exposure duration per day (8 h/day); FD=Conversion factor for working days per occupational year (250 days/year); FH=Conversion factor for working hours per occupational year [based on an 8-h workday, 5 days/week, 50 weeks/year] (1 year/2000 h).
When calculated, upper-bound 8-h TWA exposures for a product-specific task were compared to current and historical occupational exposure limits and guidelines for asbestos (0.1 f/cc or greater). These exposures were also categorized into one of the five exposure profile categories (0, 1, 2, 3, 4) according to the methodology set forth by the American Industrial Hygiene Association (AIHA) (Jahn et al. Citation2015, Chapter 5). Briefly, using this recommended strategy to determine compliance to an occupational exposure limit (OEL), the true 95th percentile of the exposure dataset (X0.95) is compared to varying percentages of the target OEL; specifically, X0.95≤1% of OEL (Category 0, trivial to non-existent exposure), 1%<X0.95≤10% of OEL (Category 1, highly controlled exposure), 10%<X0.95≤50% of OEL (Category 2, well controlled exposure), 50%<X0.95≤100% of OEL (Category 3, controlled exposure), and X0.95>100% of OEL (Category 4, poorly controlled exposure) (Hewett et al. Citation2006; Jahn et al. Citation2015, Chapter 5). An “unacceptable” exposure occurs when X0.95 exceeds the OEL (Hewett et al. Citation2006, p. 570). The X0.95 point estimate was calculated from the product-specific 8-h TWA datasets using AIHA’s IHSTAT© tool (AIHA, Falls Church, VA, USA). Using this tool, the available dataset was first assessed for lognormality. The data were determined to be lognormal and so the X0.95 point estimate using lognormal parametric statistics was reported.
Estimated cumulative asbestos exposures were also benchmarked against published “best estimate” no-observed-adverse-effect-levels (NOAEL) of 89–168 f/cc-years for lung cancer and 208–415 f/cc-years for pleural mesothelioma, which were based on exposure-response relationships identified among predominantly chrysotile-exposed cohorts (Pierce et al. Citation2016). In their analysis, Pierce et al. (Citation2016) specifically defined the NOAEL “as the highest estimated cumulative exposure at which no increased risk was reported” for lung cancer and mesothelioma in the cohorts evaluated (Pierce et al. Citation2016, p. 562). Additionally, in instances in which a study did not provide a risk estimate or confidence interval, Pierce et al. (Citation2016) calculated an estimate and/or associated confidence intervals based on the available data using a Fisher’s exact test. Further, in instances in which no incidence of cancer was reported in a cohort, Pierce et al. (Citation2016) considered the NOAEL to be the highest exposure group reported in a given study. It should therefore be noted that the chrysotile NOAEL values reported by Pierce et al. (Citation2016), and relied on in the current analysis, are indicative of minimum cumulative exposures without observed effects, and “whether or not these exposures truly represent ‘thresholds’ below which effects do not occur cannot necessarily be discerned due to [underlying] study limitations” (Pierce et al. Citation2008, p. 193).
National Occupational Mortality Surveillance (NOMS) database search
The Centers for Disease Control and Prevention’s (CDC) National Occupational Mortality Surveillance (NOMS) database monitors changes in the cause of death by occupation for various industries in the U.S. The proportionate mortality ratio (PMR) Query System from the NOMS database allows users to search calculated PMRs by cause of death and either occupation or industry, with ratios adjusted for age and, if desired, stratified by race and sex. Separate PMR search engines are available for the time period from 1985 to 1998, and for the combined time period of 1999, 2003–2004, and 2007–2014.
In a NOMS PMR query (accessed 27 November 2019) of both time periods as described above, the following parameters were selected: both “Black” and “White” for race; both “Female” and “Male” for sex; “18–64”, “65–90”, and “18–90” for age group; “Dentists”, “Medical, Dental, Optical Lab Technicians”, “Dental Hygienists”, and “Dental Assistants” for occupation; and “MN Trachea, Bronchus and Lung”, “MN Peritoneum and Pleura”, “MN Pleura (80% Mesothelioma in Males)”, “MN Mesothelioma (no codes before 1999)”, “Pneumoconioses (Occupational Lung Diseases) (Coal, Asbestos, Silica, Dust, Cotton, Other)”, and “Asbestosis” for cause of death.
Results
Product use
CRL and investment material
As briefly mentioned, asbestos-containing CRL was historically used in dentistry during the “lost-wax” casting method. This process, which is still utilized today, but without the use of asbestos CRL, is used to create various dental prostheses. According to this method, a wax model is first hand-crafted, mounted onto a base by a sprue wire, and surrounded by a metal ring (). When asbestos CRL was used, it was specified that the dental professional should cut a piece of CRL, wet it for approximately one minute, and form it around the inside of the metal ring, as demonstrated in (Taggart Citation1907; Coleman Citation1928; Brennom Citation1973). The use of a full-length “soft cushioning” asbestos CRL, situated flush with the end of the metal ring, greatly increased the accuracy of the molds by allowing the investment material to undergo a full cycle of thermal expansion (Taylor et al. Citation1930; Hollenback and Rhoads Citation1960; Hollenback Citation1964, p. 28; Shell Citation1968a, Citation1968b, Citation1969; Swartz Citation1969; Ohno et al. Citation1982; Morey Citation1992a, Citation1992b; Shibuya et al. Citation1997). The asbestos CRL, therefore, allowed for the proper expansion of the investment material during the casting process in order to ensure the proper size and physical properties of the metal casting (Souder and Paffenbarger Citation1942; J. F. Jelenko & Co. Citation1963; Skinner Citation1963). Asbestos-containing CRL was not required if a ring was not used or if a rubber ring was used in place of a metal one (Shell Citation1968a).
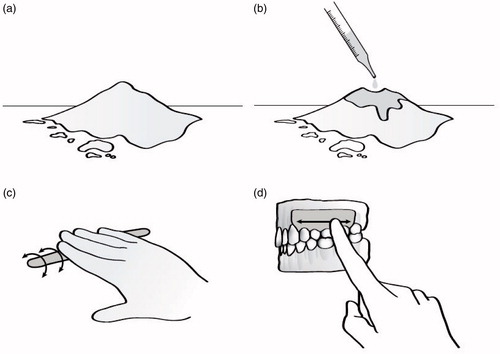
Figure 1. Per the “lost-wax” casting method, a wax model is first hand-crafted, mounted onto a base by a sprue wire, and surrounded by a metal ring (a). A piece of asbestos casting ring liner is cut, wetted for approximately one minute, and formed around the inside of the metal ring (b). Investment powder is then mixed with water to the desired consistency, poured around the wax model inside of the casting ring (with the ring liner in place), vibrated to ensure complete filling of the ring, and then allowed to harden for approximately 30 min to one hour (c and d). After the investment material hardens, the wax is subsequently burned out of the investment material in a heat treatment process lasting from 45 min to two hours, and reaching temperatures of approximately 250–900 °C (approximately 500–1650 °F), depending upon the composition of the investment material (e). Molten metal, typically gold, is then used to fill the negative space left by the burned out (or “lost”) wax in order to form the desired dental fixture (f). The invested casting is cooled for approximately six minutes at room temperature prior to being quenched in a cold-water bath, allowing for further setting of the investment material; the investment material and casting ring liner are subsequently cleaned from the final product with an abrasive sandblaster or a hand-finishing tool (g and h).
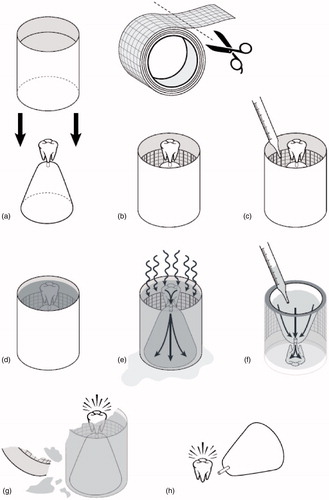
During the next step in the “lost-wax” casting process (), investment powder is mixed with water to the desired consistency, poured around the wax model inside of the casting ring (with the CRL in place), vibrated to ensure complete filling of the ring, and then allowed to harden for approximately 30 min to one hour (Moyer Citation1959; Dinger Citation1963; J. F. Jelenko & Co. Citation1963; Brennom Citation1973). Brennom (Citation1973) suggested that after filling the casting ring with investment material and while the material initially set, an approximately two-inch square piece of asbestos liner could be placed on top of the ring; however, this step may not have been performed consistently.
After the investment material hardens, the wax is subsequently burned out of the investment material in a heat treatment process () lasting from 45 min to two hours, and reaching temperatures of approximately 250–900 °C (approximately 500–1650 °F), depending upon the composition of the investment material (Taylor et al. Citation1930; Anderson Citation1956; Moyer Citation1959; Brecker Citation1961; Hollenback Citation1962; J. F. Jelenko & Co. Citation1963; Shell Citation1968a; Cutright et al. Citation1980; Brune and Beltesbrekke Citation1981; Yli-Urpo et al. Citation1982; Lacy et al. Citation1983; Takahashi et al. Citation1983; Rom et al. Citation1984; Morey Citation1991a, Citation1991b). Notably, it has been demonstrated that chrysotile is progressively converted to predominantly forsterite beginning at 400 °C (approximately 750 °F) with complete conversion at temperatures of ≥700 °C (approximately ≥1300 °F) (Dellisanti et al. Citation2001–2002).
Molten metal, typically gold, is then used to fill the negative space left by the burned out (or “lost”) wax in order to form the desired dental fixture (). The invested casting is cooled for approximately six minutes at room temperature prior to being quenched in a cold-water bath, allowing for further setting of the investment material (Dinger Citation1963). The investment material and CRL are subsequently cleaned from the final product () with an abrasive sandblaster or a hand-finishing tool (Delgado and Peyton Citation1953; Hollenback Citation1962; Dinger Citation1963; Hollenback Citation1964; Rom et al. Citation1984). Chrysotile asbestos fibers would have likely been converted to other magnesium silicates during the heat treatment process, resulting in minimal, if any, asbestos exposure to the dental professional who divested the casting (Tulachka et al. Citation1987; Dellisanti et al. Citation2001–2002).
As noted previously, the use of asbestos-containing CRL ceased by 1987, as alternative materials became more readily available (Burnett Citation1976; Priest and Horner Citation1980; Yli-Urpo et al. Citation1982; Brantley et al. Citation1986; Naylor et al. Citation1987; Earnshaw Citation1988; Morey and Earnshaw Citation1992). Yet, investigators expressed doubt as to the safety of ceramic liners, for instance, as an alternative, since “[f]ibers from ceramic ring liners closely resemble the fibers of blue [crocidolite] and brown [amosite] asbestos in size and shape and should be expected to behave similarly in depth of penetration and migration in human lungs” (Davis Citation1987, p. 368; Naylor et al. Citation1987; Tulachka et al. Citation1987). Shortcomings also exist in the physical properties of these substitute materials. Cellulose CRLs, for example, are prone to degradation at the high burnout temperatures required for “lost-wax” casting, and ceramic liners are hydrophobic (Davis Citation1995).
PDP
Until 1976, asbestos-containing PDP was used in the U.S. to heal patients’ wounds following periodontal surgery (Dyer Citation1967; Council on Dental Therapeutics [CDT] Citation1976; Cook Citation2008; Baghani and Kadkhodazadeh Citation2013). As demonstrated in , PDP was mixed with eugenol, rolled into a “sausage shape”, and applied to a patient’s gums (Cook Citation2008, p. 224). These dressings, once applied, assisted with the healing process by protecting the wound from physical trauma and providing stability at the surgical site (Baghani and Kadkhodazadeh Citation2013). Yewe-Dyer (Citation2008) reported that after his 1967 article, which described the potential asbestos-related health hazards associated with the use of PDP, was published, the use of asbestos-containing PDP in dental schools throughout the U.K. “ceased almost immediately” (Dyer Citation1967; Yewe-Dyer Citation2008, p. 544).
Bulk product analyses
Regarding the asbestos content of various dental products, three studies and one unpublished report were identified for CRL, one unpublished report for investment material, and five studies for PDP.
Generally, study samples were analyzed according to differing methods, specifically transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy-dispersive X-ray microanalysis (EDS), polarized light microscopy (PLM), X-ray powder diffraction (XRD), and standard, direct preparation procedures (AHERA/ASTMD6281/ISO10312) in conjunction with the Addison and Davies procedure. Each of these methods have distinctions in the way asbestos fibers are detected or counted that need to be considered when interpreting and comparing the results reported in each study.
CRL
Bulk product analyses indicated that CRL contained 40–95% chrysotile, and potentially trace levels (≤0.1%) of amphiboles (specifically, tremolite and actinolite) ().
Table 1. Summary of asbestos bulk analysis data for casting ring liner.
Cutright et al. (Citation1980) analyzed asbestos CRL particle debris by SEM and EDS following tearing and heating. The authors reported that chrysotile asbestos fibers were identified in samples taken with the use of acetate tape from the thumbs and index fingers of the two laboratory workers who tore the asbestos CRL. Chrysotile was also identified in samples collected from a bench top upon which investment molds were separated from asbestos-lined rings, as well as from a wall adjacent to the bench top, a casting machine, and a drawer where the asbestos CRL was stored. The authors did not quantify the asbestos content of any of these samples.
Sichletidis et al. (Citation2009) performed XRD and SEM analyses on a sample of CRL and identified chrysotile as the “exclusiv[e]” fiber type (Sichletidis et al. Citation2009, p. 929). A percent asbestos content was not reported for this sample of CRL.
In an unpublished report, Millette (Citation2013) reported bulk analysis results for three samples of CRL. Specifically, the three samples were described as (1) “Roll of asbestos tape used as a soldering block. Approx. 6.5 inch diameter metal can. Approx. 15/16 to 1 inch high”, (2) “Piece of asbestos tape approx. 3 inches long by 1.25 in wide. Labeled ‘Asbestos flask liner’”, and (3) “Roll of off-white fibrous tape on sheet metal base” (Millette Citation2013, p. 4). The samples were previously analyzed by PLM (method not reported) and later analyzed by TEM following an Addison and Davies acid/base digestion procedure and standard, direct preparation procedures (AHERA/ISO10312). For these three samples, the authors reported a range of 60–90% chrysotile by PLM and 0.0002–0.02% amphibole (tremolite and actinolite) by TEM; the habit of the amphibole minerals was not specified.
Furthermore, Millette et al. (Citation2019) published bulk analysis results for 10 separate CRL samples. The authors reported that the CRL samples were initially analyzed by stereomicroscope and that all of the samples displayed a “felted nature … with probable asbestos fibers protruding from the surface. None of the samples showed evidence of deterioration or brittleness” (Millette et al. Citation2019, p. 102). One sample of CRL was further tested for friability in a glovebox. The authors also reported that all 10 CRL samples were additionally tested for fiber releasability from the surface of the product, which was determined by SEM analysis after touching the product and then pressing the same finger onto “an adhesive-coated SEM stub” (Millette et al. Citation2019, p. 102).
Regarding the bulk analyses, all 10 samples were analyzed by PLM, which “generally follow[ed] the analytical procedures recommended by the U.S. Environmental Protection Agency for bulk building materials” (Millette et al. Citation2019, p. 102). The authors also used an Addison and Davies acid/base digestion procedure to “eliminate the soluble fractions” in order to “concentrate possible amphibole material for analysis on one sample” (Millette et al. Citation2019, p. 102). After drying, the filters were prepared for TEM analysis using standard, direct preparation procedures (AHERA/ASTMD6281/ISO10312); the authors noted that “amphibole asbestos fibers were counted according to the standard methods if [the fibers] were longer than 0.5 µm in length and had at least a 5:1 aspect ratio and substantially parallel sides” (Millette et al. Citation2019, p. 102).
Millette et al. (Citation2019) concluded that the results of the bulk analyses ranged from 40–95% chrysotile by volume by PLM with “small amounts of amphibole asbestos” identified by TEM, which were identified as “primarily tremolite” and also a “few actinolite fibers” (Millette et al. Citation2019, p. 105). Whether the amphibole fibers identified were of the asbestiform mineral habit, however, was not specified. The authors stated that “[a]ll the amphibole asbestos fibers were found by TEM after acid/base digestion”, explaining that the products they sampled contained Canadian chrysotile and may have therefore been “contaminated with amphiboles” (Millette et al. Citation2019, p. 105–106). The authors also stated that they were able to crush one of the CRL samples “into dust with hand pressure”, and therefore concluded that CRL “is a friable material that can release asbestos fibers readily” (Millette et al. Citation2019, p. 106). Quantitative data regarding their SEM analyses was not provided, however.
Investment material
Asbestos contents for investment material, or lack thereof, are summarized in .
Table 2. Summary of asbestos bulk analysis data for investment material.
As explained previously, some investment materials may have contained asbestos prior to July 1930, when the ADA’s specification for dental inlay casting investment was approved by the U.S. NBS (Broomell Citation1908; Souder and Sweeney Citation1930; Taylor et al. Citation1930). This specification detailed the material and performance requirements for investment material, and did not include any mention of asbestos with specific regard to investment material content (Souder and Sweeney Citation1930). Following a survey of 39 investment material samples, one sample was reported to contain 58.5% insoluble matter (silica and other siliceous materials) and a single “asbestos fiber” (Taylor et al. Citation1930, p. 2267). This sample was classified as a soldering investment by the manufacturer and was determined to not meet the ADA’s dental use specifications.
Further, in a 2012 unpublished report by MVA Scientific Consultants, a sample of “Dental Casting Plaster” did not contain any detectable asbestos when analyzed by PLM following EPA Method 600/R-93/116 (Compton and Millette Citation2012, p. 2).
PDP
Various bulk product analysis studies indicated a range of 3–14.29% chrysotile in PDP ().
Table 3. Summary of asbestos bulk analysis data for periodontal dressing powder.
Guglani and Allen (Citation1965) noted that a basic zinc oxide preparation of a PDP contained “6 parts asbestos” (Guglani and Allen Citation1965, p. 19). In an animal toxicity study performed by Legan et al. in Citation1973, the PDP used by the investigators contained 7.5% asbestos by weight. While the asbestos fiber type was not specified, 15% of the particles in the PDP were less than 10 µm in diameter (Legan et al. Citation1973). PDP evaluated by Otterson and Arra (Citation1974) contained 7.1%–12.8% asbestos by weight. The asbestos content for one of the samples was not quantified, however, and the asbestos fiber type was not provided for any of the PDP samples (Otterson and Arra Citation1974).
Bakdash and Frydman (Citation1976) conducted a survey to identify the types of PDP used by dental professionals in the Minneapolis-St. Paul, Minnesota, area. The authors found that of 13 dentistry practices surveyed, six exclusively used PDP that contained asbestos (fiber type not specified), four used PDP that did not contain asbestos (for “environmental reasons” or “reasons unrelated to the presence of asbestos”), and the remaining three used a combination of asbestos- and non-asbestos-containing PDP (Bakdash and Frydman Citation1976, p. 62). On average, the practices using asbestos-containing PDP each used approximately 3600 g of powder per year. Three distinct PDP products were identified and were reported to have contained 3, 7.1, and 14.29% asbestos by weight; the authors calculated that at these percent by weight contents, a total of approximately 110, 255, or 510 g of asbestos, respectively, were used in these practices per year. The authors concluded that dental professionals using asbestos-containing PDP “may be exposed to considerable quantities of asbestos” and recommended various precautions, such as the use of surgical gloves and masks, not shaking the PDP prior to mixing, and mixing the powder in a well-ventilated area (Bakdash and Frydman Citation1976, p. 63).
Fulton et al. (Citation1989) reported that the PDP used in their animal toxicity study (described in further detail below) contained 7.1% “[s]hredded [a]sbestos” by weight, per the manufacturer (Fulton et al. Citation1989, p. 195). The authors identified the asbestos fibers as chrysotile using SEM with EDS.
Animal inhalation toxicity studies
No animal inhalation toxicity studies evaluating potential health effects associated with exposure to asbestos-containing CRL or investment material were identified. It should be noted, however, that inhalation exposure to chrysotile in animals has been extensively investigated (Agency for Toxic Substances and Disease Registry [ATSDR] Citation2001). Two studies were identified in which the potential toxicity of asbestos-containing PDP following inhalation was evaluated in animals.
PDP
Acute toxicity of PDP was assessed by exposing 18 male mice to PDP (concentration and asbestos fiber type unspecified) via inhalation by a particle nebulizer for approximately four minutes (n = 6 animals), eight minutes (n = 6), or 12 min (n = 6) on a daily basis for five days; the powder was not pulverized prior to initial exposure (Legan et al. Citation1973). No histopathological changes were observed on exposure days one, two, or three. A cellular response was observed on day four in all subgroups, and the response was “greater” on day five (Legan et al. Citation1973, p. 912). The histopathologic tissue response consisted of “marked hyperplastic change in the nuclei of the cellular walls of the small terminal ducts and bronchioles associated with small localized inflammatory exudates” (Legan et al. Citation1973, p. 913). The cellular changes were confined to the bronchiolar-alveolar epithelial cells and consisted of altered morphology and staining characteristics of enlarged hyperchromatic nuclei, increased nuclear-cytoplasmic ratio, and abnormal nuclear shapes and sizes, which were reported to all be “indicative of the dysplastic process” (Legan et al. Citation1973, p. 913).
Fulton et al. (Citation1989) exposed 72 mice to 11 g of nebulized asbestos-containing PDP (referred to by the authors as Periodontal Pack Powder, PPP) for 12 min, twice a week, for a maximum period of four weeks, followed by eight weeks of no exposure; an additional 50 animals were exposed to non-asbestos-containing PDP and 43 animals were utilized as non-exposed controls. The exposure chamber had a capacity of 61.8 L, resulting in an air concentration of 178 mg/m3 of PDP, which could have possibly resulted in lung overload among the treated animals, though Fulton et al. (Citation1989) did not explicitly report such an observation. The investigators observed early interstitial pneumonia in both treatment groups, as well as a generalized resolution of the pneumonia later on in the no exposure period. In the asbestos-exposed animals, residual diffuse interstitial fibrosis, persistence of asbestos fibers within tissue, and early asbestos body formation were noted. The investigators concluded that the asbestos contained within the PDP was respirable, and suggested that dentists who have used this product “should consider themselves to have been exposed to asbestos” (Fulton et al. Citation1989, p. 199).
Fiber release studies
One study, summarizing the results of four separate fiber release analyses involving the manipulation of asbestos-containing CRL, was identified. Studies evaluating asbestos fiber release potential of investment material or PDP were not identified.
CRL
Millette et al. (Citation2019) reported airborne fiber concentrations measured during various fiber release (i.e. sealed glovebox) studies. According to the authors, airborne fiber concentrations ranged from 0.33–4.0 f/cc by phase contrast microscopy (PCM), following TEM adjustment. It should be noted that the authors reported some samples as a range even though the samples were collected during the same sampling event. Thus, for the purpose of this assessment, we averaged the two reported airborne fiber concentrations (when reported as a range for an individual study), which resulted in an overall airborne fiber concentration range of 0.33–3.1 f/cc by PCM, following TEM adjustment ().
Table 4. Summary of glovebox fiber release data for casting ring liner.
It should be noted that the glovebox and handling practices used in these fiber release analyses are not representative of the occupational environment and practices typically associated with CRL material. Specifically, samples collected from a sealed glovebox measure the airborne fiber concentrations at the source of release, and not in the breathing zone of someone performing the task (i.e. glovebox studies are designed to produce maximum peak concentrations). Thus, the sealed gloveboxes used by Millette et al. (Citation2019) are not representative of a laboratory environment, as they do not necessarily account for ventilation rates, room size, or air movement. For example, the gloveboxes were reported to have a volume of 365 L (approximately 13 ft3), whereas exposure simulations (as described in the exposure studies detailed below) were conducted in room sizes, when reported, of up to approximately 1590 ft3 (Davis Citation1995), assuming a ceiling height of eight feet. The results of these glovebox fiber release analyses were, therefore, not considered in our current exposure assessment.
Exposure studies
A total of eight asbestos exposure studies involving CRL (n = 6) and PDP (n = 2) were identified. One exposure study regarding CRL use (Jung Citation2011) was written in Korean and was translated into English prior to inclusion in our analysis. There were no studies identified in which potential asbestos exposures associated with the use of investment material were evaluated.
CRL
Airborne fiber concentrations during the manipulation of asbestos-containing CRL are summarized in . One personal sample was collected during CRL placement over 60 min and one area sample was also collected during CRL placement over 480 min (Tulachka et al. Citation1987). Notably, no fibers were detected by SEM in either sample and results were reported as <0.01 f/cc. During combined tearing and placement of CRL, personal airborne fiber concentrations ranged from 0.061 f/cc (duration: 30 min) to 3.5 f/cc (duration: 3 min) by PCM for nine task-based samples and from 0.008 to 0.0251 f/cc by PCM (duration: 240–420 min) for three longer-term samples; area concentrations ranged from <0.07–0.7 f/cc by PCM (duration: 3–6 min) for five task-based samples and 0.005 f/cc (duration: 90 min) for two longer-term samples (Davis Citation1995; Millette Citation2005c; Compton and Millette Citation2010b, Citation2010d, Citation2011a, Citation2011c; Jung Citation2011; Millette et al. Citation2019; Sichletidis et al. Citation2009). It should be noted that the NIOSH 7400 PCM results for personal air concentrations reported by Millette et al. (Citation2019) were adjusted based on their NIOSH 7402 TEM results.
Table 5. Summary of exposure study data for casting ring liner – all tasks.
Finally, personal airborne fiber concentrations during dismantling, when sampling durations were reported, ranged from 0.0046–0.0067 f/cc, on average, by PCM (duration: 420 min), and one area concentration of <0.01 f/cc by SEM over 480 min was reported (Tulachka et al. Citation1987; Jung Citation2011).
Brune and Beltesbrekke (Citation1981) measured airborne fiber concentrations during the dismantling of dental molds associated with the gold casting process. Although the study was conducted in Norway, the authors noted that an asbestos-containing CRL from the U.S. was used. During the casting procedure, the CRL allowed for expansion of the investment material during heat treatment at approximately 850 °C (approximately 1600 °F). The authors reported that the CRL becomes “brittle” at this temperature (Brune and Beltesbrekke Citation1981, p. 113). Air samples were collected on a membrane filter placed approximately 30 cm away from the workpiece during and following mold dismantling, then analyzed by light microscopy. The authors reported that dismantling “may account for a time of about 5 min.”, although they did not specify the sampling duration (Brune and Beltesbrekke Citation1981, p. 115). It was noted that there was no access to local ventilation during the procedure. Concentrations of 21 and 27 f/cc were reportedly measured during two dismantling procedure events. According to the study, fiber concentrations declined to levels less than 2 f/cc approximately 10 min after the dismantling procedure ended. Because no further information was provided on the fiber counting protocol or sampling durations, it is not possible to compare these values to current or historical occupational exposure limits, or to health-based, no-effect levels for asbestos.
Tulachka et al. (Citation1987) measured the concentration of airborne fibers generated from ceramic ring liners during simulated dental laboratory conditions and used an asbestos-containing CRL as a “control liner” (Tulachka et al. Citation1987, p. 108). The CRLs were manipulated according to the manufacturers’ instructions and were in their “usual wet condition” (Tulachka et al. Citation1987, p. 110). A single strip of CRL measuring six inches by two inches was fitted to the casting ring using finger pressure for three minutes, and then the ring was filled with investment material. The ring was heat treated to 1400 °C (approximately 2600 °F), then “water-quenched” before the investment and CRL were broken into fragments (Tulachka et al. Citation1987, p. 108). This heat treatment temperature is much higher than the temperature range described previously. Air samples were collected using membrane filters and fibers were counted using an “optical microscope … according to the rules used by the Occupational Safety and Health Agency (OSHA) for monitoring asbestos” (Tulachka et al. Citation1987, p. 109). A high-volume pump collected personal air samples “adjacent to the work space” at a rate of 23 L/min for one hour during the placement procedure and for one hour during the breakout procedure (Tulachka et al. Citation1987, p. 109).
Additionally, area samples were collected with a low-volume pump at a rate of 2 L/min over an 8-h period. Samples found to contain fibers were further analyzed using SEM. The fiber concentrations reported in the study were from the SEM results only, not the optical microscopy results. Personal fiber concentrations of less than the analytical limit of detection (LOD), or <0.01 f/cc, were reported during asbestos CRL placement. No fibers were identified during the optical microscopy analysis of the personal breakout samples, so there was no SEM analysis and no further quantitative fiber concentrations reported for these samples. Area fiber concentrations of <0.01 f/cc were reported during placement and breakout. However, the authors noted that fibers were detected in the baseline samples prior to breakout, suggesting that residual fibers remained in the air from the placement task, which occurred the day prior, such that “one cannot conclude that the liner break-out actually produced fibers” (Tulachka et al. Citation1987, p. 110).
Davis (Citation1995) evaluated the concentration of asbestos fibers released into the air during manipulation of asbestos-containing CRL material for dental castings. For each of three 30-min sampling events, pieces of the dry CRL measuring approximately 90 mm long, 38 mm wide, and 0.6 mm thick were torn from rolls and manually adapted to the inside circumference of 10 metal casting rings. The CRLs were then removed and disposed of, and the process was repeated for a total of 30 min. During the first sampling event, 80 CRL manipulations were performed (referred to as “extreme manipulation” by the author); the number of manipulations performed in the second and third sampling events was not reported (Davis Citation1995, p. 297). The author noted that in a true laboratory environment, the CRLs would have been wetted after they were placed in the rings rather than subjected to further manipulation via removal and disposal. The simulation was reportedly conducted in a 3.9 m long by 4.8 m wide (approximately 18.7 m2) laboratory under poor ventilation conditions. Personal samples were collected at a flow rate of approximately 2.4 L/min and analyzed according to NIOSH 7400, A counting rules, as well as by TEM according to the EPA Interim Transmission Microscopy Method (1987), and by EDS. By PCM analysis, personal fiber concentrations were reported to range from 0.061 (± 0.044) to 0.092 (± 0.051 or 0.065) f/mL (i.e. f/cc). The author calculated 8-h TWA concentrations of 0.004–0.006 f/mL from these values. Furthermore, the author reported that in each of the three sampling events, the airborne fiber concentration associated with manipulation of a single CRL was 0.001 f/mL. When analyzed by TEM and EDS, the fibers in the three 30-min personal samples were identified as chrysotile in concentrations of 0.200 structures ≥5 µm/mL, 0.025 structures ≥5 µm/mL, and <0.025 structures ≥5 µm/mL. The author further noted that upon examination of all the breathing zone filters, only one of 28 contained a “true, free fiber” representing 3.6% of the total material (Davis Citation1995, p. 297). As such, a maximum value for fiber concentration “to maximally assess the potential risk of mesothelioma” was calculated to be 0.0005 f/mL. In addition, area samples were collected from “across the laboratory bench away from the investigator” at an unspecified distance (Davis Citation1995, p. 295). These samples were taken over 90 min prior to the start of the experiment and again for 90 min during the experiment at a flow rate of 10 L/min. By PCM, both area fiber concentrations were 0.005 (± 0.005 to 0.006) f/mL, and 8-h TWA concentrations of 0.001 f/mL were calculated by the author for both samples. It was therefore concluded that “the concentration of asbestos fibers generated was too low to present any reasonable risk for mesothelioma induction from the manipulation of asbestos ring-lining material used in a normal manner” (Davis Citation1995, p. 296–297).
Sichletidis et al. (Citation2009) performed an air sampling survey during the manipulation of dental tape (i.e., CRL) as part of the casting procedure. Air samples were collected on membrane filters and analyzed by PCM “as described by the Asbestos International Association/RTM 1” (Sichletidis et al. Citation2009, p. 929). An air pump operating at a flow rate of 1 L/min was set 15–20 cm above the work bench at approximate breathing level. The authors noted that “[t]he experiment took place in a room with approximately the same surface area (about 5 m2) as the dental laboratories used by the three dentists”, who were the subjects of the authors’ case series included in this study, which is described in greater detail below (Sichletidis et al. Citation2009, p. 928–929). There was reportedly no ventilation in the room. The authors stated that “[f]irst, pieces of paper that contained asbestos were torn from dental tape rolls and then placed into water; a mold was shaped using the mass of wet asbestos. The structure was dried out in a furnace. Then a piece of gold was put in the asbestos mold” with borax and subsequently burned out (Sichletidis et al. Citation2009, p. 929). The procedure was repeated continuously for four hours, representing the approximate time that dentists worked in their laboratories each day. The total number of CRL tears or molds produced, however, was not quantified. By PCM, airborne fiber concentrations were 0.008 f/cc during the 4-h sampling period. The authors noted, however, that “it is possible that the actual concentrations of fibers in the atmosphere of a small enclosed laboratory were greater than those achieved in [their] experiment of only 4 h” (Sichletidis et al. Citation2009, p. 930).
Jung (Citation2011) conducted air sampling in 10 dental laboratories across Daegu, South Korea, between December 2010 and January 2011. Air sampling and analysis were conducted according to NIOSH 7400. Briefly, air samples were collected on a 25-mm cellulose ester membrane filter using an open-face, three-piece cassette with an extension cowl. The pump was set to a flow rate of 1.7 L/min. Samples were collected on top of a workbench (approximately 1–1.5 m from floor) over a period of approximately seven hours, and were analyzed by PCM. The investigators collected a total of 40 air samples (four per site); however, only 32 of the 40 samples were collected during the use of asbestos-containing (fiber type not specified) CRL. Half of the samples (n = 16) were collected in a room during preparation in which the dental laboratory technician tore or cut CRL, inserted it inside a metal ring containing a wax model, and then filled in the ring with plaster, which was allowed to dry prior to being burned out in a furnace. The other half of the samples (n = 16) were collected in a second room where burnout and plaster removal occurred. It was estimated that burnout occurred one to two times per day on average, and two to four prosthetics were produced for each burnout. Average (geometric mean) airborne fiber concentrations ranged from 0.0162–0.0251 f/cc by PCM in the preparation room, and from 0.0046–0.0067 f/cc by PCM in the burnout room. It was noted that there was natural ventilation (i.e., windows), as well as local exhaust ventilation in the burnout room, which the authors hypothesized contributed to the lower air concentrations in the burnout room compared to the preparation room; ventilation in the preparation room was reportedly described as insufficient.
Millette et al. (Citation2019) reported the results of six separate chamber studies involving the handling, cutting/tearing, and placement of CRL into metal cylinders. Although the authors did not cite any of the unpublished reports upon which their analysis was based, we were able to identify all but one (the bulk analysis detailing sample ID 11081-AA1451) of these underlying reports (Compton and Millette Citation2010a, Citation2010b, Citation2010c, Citation2010d, Citation2011a, Citation2011b, Citation2011c, Citation2011d, Citation2011e, Citation2012; Millette Citation2004, Citation2005a, Citation2005b, Citation2005c, Citation2005d, Citation2008). Regarding the chamber studies detailed by Millette et al. (Citation2019), the authors did not report any of the area sample results for these experiments; however, these data were included in their unpublished reports, so we have chosen to present these data in our analysis and these results are detailed below. Further, Millette et al. (Citation2019) reported air concentrations as ranges in their published study; however, based on the respective underlying unpublished reports, these air samples do not appear to be independent samples, but rather samples that were collected simultaneously on both lapels of the individual performing the given task. We therefore averaged the two reported airborne fiber concentrations in our analysis whenever Millette et al. (Citation2019) presented them as a range.
Two of the chamber tests entailed the “wet press” of CRL after tearing (Millette et al. Citation2019, p. 103). The test chamber was approximately 9 feet high by 10 feet wide by 12 feet long, and had a HEPA-equipped device that was used to clean the area before the tests. The HEPA unit was also operational during the studies at a flow rate of 45 cubic feet per minute (CFM), which was equal to approximately 2.5 ACH. Personal air samples were collected on “standard” air filter cassettes at flow rates of 1–5 L/min, and were analyzed by PCM (NIOSH 7400) and TEM (NIOSH 7402); one air sample was also analyzed according to the AHERA TEM method (Millette et al. Citation2019, p. 105). Personal air concentrations ranged from 0.69–3.5 f/cc by PCM, following TEM adjustment (). It was noted that the “PCM fibers counted in the air samples contained primarily chrysotile asbestos with one exception. One tremolite asbestos bundle was found in one of the air sample[s] collected during [one of the tests]. This bundle was 5.7 µm long and 0.38 µm wide” (Millette et al. Citation2019, p. 107). Notably, however, in the underlying unpublished report in which this “tremolite asbestos bundle” was initially reported, Compton and Millette (Citation2010b) stated that this “bundle” was identified in the area sample collected while the tape was being torn. As noted, Millette et al. (Citation2019) made no reference to any area samples collected from the studies. In addition, one “bundle of tremolite asbestos fibers” was also reported to have been identified in an air sample by Compton and Millette (Citation2010d), though the investigators did not specify if this bundle was found in the personal samples or area sample; further, Millette et al. (Citation2019) did not report this bundle for this specific chamber study in their Table 1.
In the five underlying unpublished reports we identified in which the investigators reported the results from area sampling during the respective chamber studies, the area sample air concentrations ranged from <0.07–0.7 f/cc by PCM (duration: 3–6 min) (Compton and Millette Citation2010b, Citation2010d, Citation2011a, Citation2011c; Millette, Citation2005c).
PDP
Potential airborne asbestos exposure associated with PDP could occur when opening a bottle containing PDP, as well as during the mixing of PDP with liquid (Dyer Citation1967; Bakdash and Frydman Citation1976; Reid et al. Citation1991). However, potential asbestos exposure from opening bottles of PDP was not well characterized in the available literature. The available studies report that breathing zone fiber concentrations during mixing of PDP ranged from <0.0044–<0.297 f/cc by PCM, while area concentrations during mixing ranged from <0.0054–<0.396 f/cc by PCM (). None of the samples in any of the studies identified resulted in detectable fiber concentrations above the LOD.
Table 6. Summary of exposure study data† for periodontal dressing powder – all tasks.
Dyer (Citation1967) collected samples during the use of gingivectomy packs (i.e., PDP). Microscope slides covered in egg albumin were placed at table height and at breathing zone height (three feet above the table) in the pack mixing area. It was noted that a chairside assistant “usually shakes the bottle containing the dry constituents of the pack so that on opening the bottle a cloud of dust is emitted” (Dyer Citation1967, p. 507). The gingivectomy packs in this study were therefore prepared with and without shaking the bottle. An unspecified concentration of asbestos fibers was reportedly observed on slides at both heights, as well as with and without shaking of the bottle, “demonstrating that the distribution of asbestos fibre in air is not dependent on shaking the bottle” (Dyer Citation1967, p. 507). The method of asbestos fiber identification was not described. Further, additional slides were introduced five minutes after pack mixing had begun, and remained in place for an additional five minutes. Asbestos fibers were not observed on these slides. Dyer concluded that a dentist and his assistant may be exposed to “considerable quantities” of asbestos during a lifetime of practice and therefore suggested that the use of gingivectomy packs containing asbestos be discontinued (Dyer Citation1967, p. 507). Subsequent investigators commented on this study, noting that “this method of evaluation deviates from the sampling procedure being used by OSHA industrial hygienists” (Otterson and Arra Citation1974, p. 436).
Otterson and Arra (Citation1974), employees of the Section of Occupational Health and Section of Dental Health of the Wisconsin Division of Health, performed three studies to evaluate exposures to airborne asbestos fibers during the preparation of periodontal packs; two studies were performed at periodontists’ offices, and the third study was conducted at a dental assisting technical college. Samples were collected on open-face membrane filters at a flow rate of 2 L/min. Fibers greater than 5 µm in length were counted using PCM; it was noted that the sampling and analytical procedures were “in accordance with [the] method and practices specified by [OSHA]” (Otterson and Arra Citation1974, p. 436).
In the first dental office, the periodontal pack powder was mixed with a “small” amount of 2% thymol in eugenol into a ball of “putty-like” consistency, which was wrapped in plastic and stored for use throughout the week (Otterson and Arra Citation1974, p. 436). The powder reportedly contained approximately 13% asbestos by weight, and the task lasted approximately 30 min. Airborne concentrations of <0.0044 and <0.0063 f/cc were reported for the two personal samples collected during mixing of 28- and 20-min samples, respectively, while one ambient air sample collected from four feet behind the technician at breathing level for 23 min resulted in an airborne concentration of <0.0054 f/cc.
In the second office, powder containing 7.1% “[s]hredded” asbestos by weight was mixed with a “small” amount of 2% thymol in eugenol (Otterson and Arra Citation1974, p. 436–437). Mixing lasted four minutes and the product was prepared in the office “as needed” rather than in advance for the week (Otterson and Arra Citation1974, p. 436). An airborne fiber concentration of approximately <0.297 f/cc was reported for the 5-min personal sample during mixing. Additionally, one ambient air sample collected from four feet behind the technician during mixing (<0.396 f/cc) was collected for four minutes.
At the technical college, three students and the instructor each simultaneously mixed a “thimbleful” of powder of unspecified asbestos content “gradually” with a “few” drops of 2% thymol in eugenol (Otterson and Arra Citation1974, p. 437). It was noted that this task typically took four minutes, but the participants were asked to continue mixing for a “minimum of 15 minutes” in this simulation (Otterson and Arra Citation1974, p. 438). Fiber concentrations were reported as <0.070 (instructor’s breathing zone sample taken over 18 min) to <0.074 f/cc (three students’ breathing zone samples taken over 17 min each) for the four personal samples. One ambient air sample taken for 23 min resulted in a fiber concentration of <0.054 f/cc. For all three studies, “not one fiber” was detected on any of the filters (Otterson and Arra Citation1974, p. 437).
Otterson and Arra (Citation1974) concluded that the “mixing and kneading of the packs with a spatula and hands does not seem to be conducive to dispersing any detectable number of fibers into the laboratory air”, indicating that there was no potential hazard from airborne asbestos fibers when periodontal packs were prepared under conditions existing during these studies (Otterson and Arra Citation1974, p. 437). However, the authors ultimately recommended that unspecified precautionary measures be taken when using PDP.
Human health studies
Mesothelioma
No cases of death or disease in dentists or dental laboratory technicians from asbestosis, mesothelioma, or bronchogenic lung cancers induced by asbestos were reported in the literature as of 1987, according to a personal communication between Drs. Davis and Selikoff, although CRL containing chrysotile had been in use for approximately 50 years by this time (Davis Citation1986, Citation1987; Earnshaw Citation1988). Various epidemiological studies have evaluated cancer risk among dentists; mesothelioma was not a disease outcome of interest evaluated in any of these studies (Eriksson et al. Citation1998; Shimpo et al. Citation1998; Simning and van Wijngaarden Citation2007; Koifman et al. Citation2014). Interestingly, in a large-scale cohort study performed from 1961–2005 across five Nordic countries including 15 million people, four cases of mesothelioma (pleural and peritoneal combined) in male dentists (Standardized Incidence Ratio [SIR] = 0.37 [95% Confidence Interval (CI)=0.10 − 0.94]) among 13,539 male dentists (contributing a total of 191,711 person-years of observation), and two cases of mesothelioma in female dentists (SIR = 1.36 [95% CI = 0.16 − 4.92]) among 8,702 female dentists (contributing a total of 215,346 person-years of observation) were reported, but no significantly increased risk was found (Pukkala et al. Citation2009). Another cohort study evaluating cancer risk by occupation and socioeconomic status among Swiss men was conducted from 1980 to 1993 (Bouchardy et al. Citation2002). Out of 58,134 incident cases of cancer, 249 cases were classified as “dentists, veterinarians, and pharmacists”. While there were no cases of pleural mesothelioma or cancer of the pleura in this group, two cases of cancer of the peritoneum were observed, resulting in a non-significant odds ratio (OR) of 2.1 (95% CI = 0.5 − 8.8) after adjusting for registry, age, civil status, period of diagnosis, nationality, and type of habitat. Upon further adjustment for socioeconomic status, an OR of 3.1 (95% CI = 0.6 − 16.8), which was also not statistically significant, was calculated.
Upon review of the available literature, a total of 12 cases of mesothelioma in dental professionals and their relatives who used and/or were bystanders to the use of potentially asbestos-containing dental products were identified (Reid et al. Citation1991; Ogus and Bourne Citation2007; Fry Citation2008, Citation2009; Markowitz and Moline Citation2017; Mensi et al. Citation2017). These cases are described in detail in .
Table 7. Summary of mesothelioma case reports among asbestos-containing dental product users.
Lung cancer
Researchers have generally concluded that dentists are not at an increased risk of lung or respiratory cancer (Glass Citation1966; Hill and Harvey Citation1972; Blair et al. Citation1985; Sankila et al. Citation1990; Rix and Lynge Citation1996; Eriksson et al. Citation1998; Haldorsen et al. Citation2004; Simning and van Wijngaarden Citation2007; Pukkala et al. Citation2009; Koifman et al. Citation2014). One study of dentists in Tokyo (Shimpo et al. Citation1998) found that standardized mortality ratios (SMR) for lung, tracheal, and bronchial cancers in this worker population were higher than in the general population of Tokyo, but these differences were not statistically significant. Nishio et al. (Citation2004) concluded that male dentists in Japan did not have a significantly different risk for lung cancer than the general population. However, the authors did not control for smoking. Instead, they noted that their subjects likely had a lower smoking rate compared to the general population, such that the occupational lung cancer risk they calculated for dentists was underestimated.
In an additional study conducted in Los Angeles, California, the investigators reviewed 2,161 death certificates from 1968–1970 that mentioned lung cancer, as well as 1,777 incident cases of lung cancer from 1972–1973, in white males aged 20–64 (Menck and Henderson Citation1976). SMRs were calculated for various occupations; dental laboratory technicians were reported to have an excess risk of lung cancer (SMR = 405), whereas dentists were not (SMR= <70). Neither confidence intervals nor p-values were provided; as such, statistical significance could not be readily determined. The investigators reported that “[m]ost of the risk to lung cancer associated with the high risk occupations and industries … may be related to exposure to asbestos or polycyclic aromatic hydrocarbons (PAH) or both”; the authors noted that dental laboratory technicians specifically were thought to have been previously exposed to PAH (Menck and Henderson Citation1976, p. 799).
In the Swiss cancer incidence study conducted by Bouchardy et al. (Citation2002), 33 cases of lung cancer were observed among “dentists, veterinarians, and pharmacists”. The authors calculated a non-significant OR of 0.8 (95% CI = 0.5 − 1.2) after adjusting for registry, age, civil status, period of diagnosis, nationality, type of habitat, and socioeconomic status. However, a statistically significant decreased risk of lung cancer (OR = 0.4; 95% CI = 0.3 − 0.6; p < 0.001) was calculated after adjusting for registry, age, civil status, period of diagnosis, nationality, type of habitat, histological confirmation of tumor, and death certificate.
Furthermore, one case report of lung cancer in a dentist was identified in the literature. Sichletidis et al. (Citation2009) described the medical histories of three Greek men who had been employed as dentists for 35–45 years. All three dentists previously used asbestos-containing dental tape during regular casting operations and had no other reported asbestos exposures apart from the potential exposures they experienced as dentists. All three of these individuals had pleural plaques. One of the patients was also diagnosed with lung cancer. The authors hypothesized that the patient’s lung cancer “may be the result of synergic action of cigarette smoking with asbestos exposure” (Sichletidis et al. Citation2009, p. 930). This individual had a smoking history of 80 pack-years.
Nonmalignant respiratory disease (NMRD)
The prevalence of pneumoconiosis in domestic and international populations of dental laboratory technicians has been extensively characterized, and the generally increased risk of pneumoconiosis and decreased lung function observed in this worker population is thought to be caused by complex dust exposures to a variety of agents, including silica, metal alloys, acrylic plastics, asbestos, and alginate powders (Tuengerthal et al. Citation1983; Morgenroth et al. Citation1985; Loewen et al. Citation1988; Barrett et al. Citation1989; Sherson et al. Citation1990; Choudat et al. Citation1993; Choudat Citation1994; Selden et al. Citation1995; Choël et al. Citation1999; Froudarakis et al. Citation1999; Nayebzadeh et al. Citation1999; Radi et al. Citation2002; Kartaloglu et al. Citation2003).
Rom et al. (Citation1984) conducted a case-control study of 178 dental laboratory technicians and 69 controls in Utah; they identified eight cases of pneumoconiosis and found that this cohort also had decreased pulmonary function compared to a non-exposed group, after controlling for smoking status. The authors noted that the cases of pneumoconiosis may have been related to prior silica or non-precious metal alloy dust exposures. They also performed full-shift personal air sampling for nickel, chromium, cobalt, beryllium, silica, and methyl methacrylate in 42 dental laboratories. Although it was noted that the production of crowns and bridges using the “lost-wax” casting method was performed at these laboratories, the authors did not sample for any potential airborne asbestos concentrations (Rom et al. Citation1984).
Sherson et al. (Citation1988) performed a case-control study of 31 Danish dental laboratory technicians. The authors identified six cases of pneumoconiosis, and noted that the 13 technicians who fabricated any type of prostheses for at least 15 years had consistently lower lung function than controls, although these differences were not significant. Silica, beryllium, chromium, or cobalt exposures were hypothesized to be causative agents; asbestos was not mentioned as a possible cause. The authors further noted that four of the 31 technicians and one of the 30 controls had small opacities (≥category 1/0), indicating an increased relative risk in dental laboratory technicians for developing these opacities (Sherson et al. Citation1988).
Tuengerthal et al. (Citation1983) performed lung biopsies on 20 dental laboratory technicians. Interstitial fibrotic lesions characteristic of pneumoconiosis were observed in 20 workers, while “asbestos fibers” were identified by energy-dispersive X-ray spectroscopy (EDXA) in one individual (Tuengerthal et al. Citation1983, p. 1207).
De Vuyst et al. (Citation1986) identified two cases of pneumoconiosis in dental laboratory technicians. The first case was a 49-year-old man who worked as a dental laboratory technician from 1949–1982. It was noted that he had biapical pleural thickening, a 3.5 pack-year smoking history, and spontaneous pneumothorax, in addition to chromium-cobalt-molybdenum alloy, silicon carbide, and asbestos exposures. Regarding his use of asbestos, it was reported that he cut “asbestos foils to coat molds during casting operations”, and used these foils to protect furniture on which prosthetic parts were ground and soldered (De Vuyst et al. Citation1986, p. 316). Whether the “asbestos foils” referenced by De Vuyst et al. were asbestos-containing CRL materials was not clear. Analysis by light microscopy and SEM with EDS of this individual’s bronchoalveolar fluid (BAF) and lung tissue revealed 22–68 asbestos bodies/mL and 160 asbestos bodies/g of wet lung, respectively; ferruginous bodies were also observed in the fibrotic areas. The authors noted, however, that the asbestos bodies detected in the lung tissue corresponded “to a low concentration that appeared insufficient to provoke asbestosis” (De Vuyst et al. Citation1986, p. 316). The second case was a 30-year-old woman who worked in a dental laboratory from 1972 until 1979, during which she was exposed to chromium-cobalt-molybdenum alloy; she had no known contact with asbestos and had a 2 pack-year smoking history. No asbestos bodies were found in her BAF, and her lung tissue was not sampled.
In 1999, Nayebzadeh et al. reported a case of pneumoconiosis in a dental laboratory technician in Quebec, Canada. The 51-year-old male worked as a dental laboratory technician for approximately 30 years prior to his diagnosis, and did not have any other employments during his lifetime. Based on a lung burden analysis, chrysotile asbestos fibers, and silica, chromium-cobalt, clay, mica, iron, and aluminum particles were observed. No amphibole asbestos fibers were identified, nor was any formation of ferruginous bodies by PCM. The authors concluded that “[t]he concentration of [chrysotile] asbestos fibers indicates occupational exposure to asbestos fibers”, and noted that chrysotile asbestos-containing materials, including CRL, were used in dental laboratories in Quebec (Nayebzadeh et al. Citation1999, p. 351). However, the authors acknowledged that the use of CRL during casting “did not involve significant generation of dust from the asbestos sheet” (Nayebzadeh et al. Citation1999, p. 353). Yet, they did not provide any additional explanation for the presence of occupational levels of chrysotile in this patient’s lungs.
Kartaloglu et al. (Citation2003) also identified two cases of pneumoconiosis in dental laboratory technicians who each worked in a laboratory for eight years prior to their respective diagnoses. The authors noted that “the typical radiological appearance of asbestosis was absent and asbestos was not detected by mineralogical examination” (Kartaloglu et al. Citation2003, p. 172).
Other nonmalignant respiratory conditions apart from pneumoconiosis have also been described in dental professionals and their family members. Sichletidis et al. (Citation2009) described three cases of pleural plaques in Greek dentists. In addition to the lung cancer case reported by these authors, as noted above, a patient with obstructive lung disease was also identified. The authors attributed this patient’s lung disease to an extensive smoking history. In a case series published by Markowitz and Moline in 2017, two of the patients were diagnosed with pleural plaques and an additional two were diagnosed with pleural thickening (Markowitz and Moline Citation2017). Three cases of pleural thickening, as well as one case of fibrosis, were reported by Mensi et al. (Citation2017) in their case series. In a survey of 731 male and female Swedish dental laboratory technicians, 224 individuals reported experiencing respiratory tract irritation and reactions; the authors also identified one case of “pleuratic plaque”, which they attributed to asbestos exposure (Jacobsen et al. Citation1996, p. 140).
NOMS database search
The NOMS PMR Query System search indicated that the PMR for malignant neoplasms of the trachea, bronchus, and lung in medical, dental, and optical lab technicians was significantly (p < 0.05) elevated for white females aged 65–90 (PMR = 139; 95% CI: 110 − 175) and for white females aged 18–90 (PMR = 127; 95% CI: 105 − 152) from 1999, 2003–2004, and 2007–2014. The statistically significant findings for neoplasms of the trachea, bronchus, and lung were not adjusted for smoking status. In white male dentists, the PMR for malignant neoplasms of the trachea, bronchus, and lung was significantly (p < 0.01) decreased for all age groups and all time periods. Furthermore, the PMR for pneumoconioses in dentists was significantly (p < 0.01) decreased in white males aged 65–90 (PMR = 59; 95% CI: 38 − 87) and in white males aged 18–90 (PMR = 59; 95% CI: 38 − 86) from 1999, 2003–2004, and 2007–2014. Notably, the PMRs for mesothelioma and asbestosis in all occupations assessed (i.e., dentists; medical, dental, and optical lab technicians; dental hygienists; and dental assistants) for all time periods were not statistically significant, suggesting that these occupations are not at an increased risk of developing these diseases.
Exposure assessment
Personal and area (bystander) exposure scenarios were evaluated to characterize potential asbestos exposures generated during the typical use of asbestos-containing CRL and PDP. The number of years of product use for each profession were based on mean employment tenures as reported by Maguire (Citation1993) for dental assistants (5.4 years), dental hygienists (10.6 years), dental laboratory technicians (12.9 years), and dentists (15.1 years). When calculated, estimated 8-h TWA concentrations for CRL and PDP are presented in and , respectively. Cumulative asbestos exposure estimates are presented in .
Table 8. Cumulative exposure estimates for various dental professionals.
CRL – placement
Tulachka et al. (Citation1987) was the only study identified that attempted to measure a personal and area airborne fiber concentration during placement of CRL. The distance at which the area sample was taken was not reported. The authors reported a concentration of <0.01 f/cc by SEM for both samples with sampling durations of 60 min for the personal sample and 480 min for the area sample. As noted previously, the entire dataset describing airborne fiber exposures potentially generated during the placement of CRL consisted of censored (i.e. non-measurable or non-detectable) results below the analytical LOD. Based on these data, any potential asbestos exposures during placement of CRL are likely low, if measurable at all, and perhaps even non-existent (i.e., zero exposure), because Tulachka et al. (Citation1987) reported using the CRL in a “wet condition”, which was standard practice (Tulachka et al. Citation1987, p. 108). Because of the fact that no fibers were detected in the only dataset available for our review, we did not estimate a potential upper-bound 8-h TWA or cumulative asbestos exposures associated with this specific task.
CRL – tearing, placement
Personal
Four exposure studies (Davis Citation1995; Sichletidis et al. Citation2009; Jung Citation2011; Millette et al. Citation2019) in which personal airborne fiber concentrations were measured during tearing with subsequent placement of CRL inside the metal casting ring were identified. Airborne fiber concentrations ranged from 0.008 f/cc (Sichletidis et al. Citation2009) to 3.5 f/cc (Millette et al. Citation2019) by PCM with sampling durations ranging from three minutes (Millette et al. Citation2019) to 420 min (Jung Citation2011). An upper-bound 8-h TWA of 0.022 f/cc (= [0.0251 f/cc × 420 min]/480 min) was therefore calculated using EquationEquation (1)(1)
(1) . It should be noted that Davis (Citation1995) tore and installed CRL 80 times in 30 min, resulting in 8-h TWA values of 0.004 f/cc (= [0.061 f/cc × 30 min]/480 min) to 0.006 f/cc (= [0.092 f/cc × 30 min]/480 min), which are approximately an order of magnitude lower than the 8-h TWA calculated from the data produced by Jung (Citation2011), in which up to eight molds (and, therefore, eight CRL tears) may have been performed during the sampling period. Based on the 8-h TWA of 0.022 f/cc, as calculated above, cumulative asbestos exposure estimates were 0.12 f/cc-years (=0.022 f/cc × 5.4 years) for dental assistants, 0.23 f/cc-years (=0.022 f/cc × 10.6 years) for dental hygienists, 0.28 f/cc-years (=0.022 f/cc × 12.9 years) for dental laboratory technicians, and 0.33 f/cc-years (=0.022 f/cc × 15.1 years) for dentists.
Area (bystander)
Seven area airborne fiber samples were collected and reported across six studies and/or reports (Compton and Millette Citation2010b, Citation2010d, Citation2011a, Citation2011c; Davis, Citation1995; Millette, Citation2005c). Compton and Millette (Citation2011c, Citation2011a) reported sampling distances of approximately five feet from the task. Davis (Citation1995), Millette (Citation2005c), Compton and Millette (Citation2010b), and Compton and Millette (Citation2010d) did not specify sampling distances for their area samples. Airborne fiber concentrations ranged from 0.005 f/cc (Davis Citation1995) to 0.7 f/cc (Compton and Millette Citation2010b) by PCM. Sampling durations ranged from three minutes (Compton and Millette Citation2010b) to 90 min (Davis Citation1995). An upper-bound bystander 8-h TWA of 0.0044 f/cc (= [0.7 f/cc × 3 min]/480 min) was calculated according to EquationEquation (1)(1)
(1) . Using this 8-h TWA, cumulative asbestos exposures for bystanders were estimated to be 0.024 f/cc-years (=0.0044 f/cc × 5.4 years) for dental assistants, 0.046 f/cc-years (=0.0044 f/cc × 10.6 years) for dental hygienists, 0.056 f/cc-years (=0.0044 f/cc × 12.9 years) for dental laboratory technicians, and 0.066 f/cc-years (=0.0044 f/cc × 15.1 years) for dentists.
CRL – dismantling
Three exposure studies (Brune and Beltesbrekke Citation1981; Tulachka et al. Citation1987; Jung Citation2011) provided data measured during the dismantling of dental molds associated with the casting process. Brune and Beltesbrekke (Citation1981) reported concentrations of 21 and 27 f/cc by light microscopy. These investigators did not specify sampling durations. Tulachka et al. (Citation1987) reported an airborne fiber concentration of <0.01 f/cc by SEM sampled for 60 min during dismantling (“break-out”). Jung (Citation2011) further reported average (geometric mean) airborne fiber concentrations during dismantling (“burnout”) ranging from 0.0046–0.0067 f/cc by PCM over a sampling period of approximately 420 min. Regarding area (bystander) sampling, Tulachka et al. (Citation1987) reported a long-term (480-min) airborne concentration of <0.01 f/cc by SEM taken from an unspecified distance during dismantling.
As noted previously, however, chrysotile in the CRL is likely to be converted to other magnesium silicates during heat treatment due to the high temperatures necessary for this process. The measurements reported in these studies are therefore unlikely to accurately reflect true airborne asbestos concentrations. Indeed, Tulachka et al. (Citation1987) stated their finding of fibers in baseline samples collected prior to dismantling suggested “the existence of residual fibers in the room air from the previous day, and thus one cannot conclude that the liner break-out actually produced fibers” (Tulachka et al. Citation1987, p. 110). Because of lack of sufficient evidence that asbestos exposures would occur during CRL dismantling, 8-h TWAs and cumulative asbestos exposures for this task were therefore not calculated.
Investment material
Investment material has not intentionally contained asbestos since, perhaps, 1930, if ever at all (Broomell Citation1908; Souder and Sweeney Citation1930; Taylor et al. Citation1930). As such, we were unable to identify any exposure studies for this product, and we did not estimate potential asbestos exposures associated with the use of this product.
PDP – bottle opening
Dyer (Citation1967) reported that asbestos fibers were measured upon opening a bottle of PDP, independent of shaking the bottle prior to opening. However, neither sampling duration, airborne fiber concentration, nor method of detection were reported for these samples. Therefore, potential asbestos exposures associated with this task were not estimated.
PDP – during mixing
Seven personal samples taken during mixing of PDP ranged from <0.0044–<0.297 f/cc by PCM (Otterson and Arra Citation1974). Sampling durations ranged from 5–28 min. Otterson and Arra (Citation1974) also reported three area samples taken during PDP mixing. The reported fiber concentrations ranged from <0.0054–<0.396 f/cc by PCM and were collected for 4–23 min from approximately four feet behind the individual performing the mixing. Again, as noted, no fibers were detected in any of the samples attempting to measure airborne fiber concentrations potentially generated during the mixing of PDP and potential exposures may, therefore, be as low as zero. Thus, we did not estimate 8-h TWAs or cumulative asbestos exposures associated with this specific task.
PDP – after mixing
Dyer (Citation1967) also reported that one personal sample taken for five minutes after the commencement of mixing PDP “did not show any asbestos fibres” (Dyer Citation1967, p. 507). Neither a quantitative LOD, nor a method of detection were provided, however, and so we were unable to estimate the potential asbestos exposure associated with time spent in the laboratory after PDP mixing tasks occurred.
Benchmark comparisons
The 8-h TWA concentrations calculated from the upper range of the available data associated with tearing followed by placement of CRL (personal: 0.022 f/cc, bystander: 0.0044 f/cc; ) were below the current OSHA Permissible Exposure Limit (PEL) and American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Value (TLV) of 0.1 f/cc for asbestos. As a result, these calculated 8-h TWA exposures would have also been much less than any historical occupational limit for asbestos, which were all greater than 0.1 f/cc. Furthermore, the X0.95 point estimate of the dataset for the calculated personal 8-h TWA concentrations for tearing followed by placement of CRL was 0.03 f/cc, which is classified as Category 2 (well controlled exposure; 10%<X0.95≤50% of the OEL) using AIHA’s methodology, and the X0.95 point estimate of the dataset for the calculated bystander 8-h TWA concentrations for the same CRL task was 0.007 f/cc, which is classified as Category 1 (highly controlled exposure; 1%<X0.95≤10% of the OEL). In other words, personal 8-h TWA exposures associated with this particular task would be expected to be below 0.03 f/cc 95% of the time, and bystander exposures below 0.007 f/cc 95% of the time.
We also sought to benchmark cumulative asbestos exposure estimates (for the products/tasks for which we were able to calculate 8-h TWA concentrations) against published chrysotile NOAELs for lung cancer (89–168 f/cc-years) and pleural mesothelioma (208–415 f/cc-years), as reported by Pierce et al. (Citation2016). Although Pierce et al. (Citation2016) did not estimate a chrysotile NOAEL for peritoneal mesothelioma, the authors reported that “if peritoneal mesothelioma is caused by chrysotile, the level of cumulative exposure required to develop the disease exceeds that associated with pleural mesothelioma” (Pierce et al. Citation2016, p. 581).
As dentists were reported to have the longest average tenure of the dental professionals discussed (15.1 years as compared to 5.4 years for dental assistants, 10.6 years for dental hygienists, and 12.9 years for dental laboratory technicians) (Maguire Citation1993), cumulative asbestos exposures for this job title were used as plausible, upper-bound values to which to compare to the published chrysotile NOAELs. The greatest estimated personal cumulative exposure associated with daily use of CRL (tearing, placement) was 0.33 f/cc-years, and the greatest area (bystander) cumulative exposure estimate was 0.066 f/cc-years (). Thus, all of the calculated cumulative asbestos exposure estimates for dental professionals are well below published chrysotile “best estimate” NOAELs for lung cancer and pleural mesothelioma (Pierce et al. Citation2016).
Even assuming an average 45-year working lifetime of exposure, the personal cumulative asbestos exposure estimate for daily use of CRL (tearing, placement) would be 0.99 f/cc-years (=0.022 f/cc × 45 years). Thus, this working lifetime cumulative exposure estimate is orders of magnitude below the published chrysotile NOAELs for lung cancer and pleural mesothelioma, as well as below a cumulative asbestos exposure at the current OSHA PEL (0.1 f/cc) for a working lifetime of 45 years (0.1 f/cc × 45 years = 4.5 f/cc-years).
Discussion
Some researchers have recently suggested that, based on case series and reports of mesothelioma among dental professionals, there exists an increased risk of mesothelioma associated with asbestos exposures from dental products (Markowitz and Moline Citation2017; Mensi et al. Citation2017). Other investigators, however, have previously concluded that any potential airborne asbestos exposures associated with the typical use of these products are too low to pose a health risk (Davis Citation1987; Tulachka et al. Citation1987; Davis Citation1995; Choël et al. Citation1999), which is supported by the consistently negative findings of mesothelioma risk among dental professionals as reported by more robust and rigorous epidemiological studies of these occupations (Bouchardy et al. Citation2002; Pukkala et al. Citation2009). Because of the current awareness of the potential health risks associated with the inhalation of asbestos fibers, we sought to quantitatively characterize potential airborne asbestos exposures experienced by dental professionals from their typical use of asbestos-containing dental products in an occupational setting, and to assess their potential risk of ARD.
This study is the first, to our knowledge, in which a comprehensive literature review was performed in order to aggregate all of the relevant data related to the asbestos content of dental products, the exposure data associated with the typical use of these products, and the available animal and human health effect data related to potential asbestos exposures from these products. These data allowed us to derive cumulative asbestos exposure estimates, which have not been previously estimated, for dental professionals who may have previously used these products. Further, because the focus of our analysis was on “OSHA-sized” fibers (i.e. fibers greater than 5 µm in length with an aspect ratio of at least 3:1), we are able compare the results of our analysis to both occupational exposure limits, and the vast exposure and epidemiology literature specific to asbestos.
Exposure assessment
For CRL tearing and placement, we calculated personal cumulative exposure estimates, based on measurable airborne fiber concentrations, of 0.12 f/cc-years for dental assistants, 0.23 f/cc-years for dental hygienists, 0.28 f/cc-years for dental laboratory technicians, and 0.33 f/cc-years for dentists. Potential personal cumulative asbestos exposures during dismantling were not estimated because of the likely conversion of chrysotile to other magnesium silicates during heat treatment. Based on entirely censored exposure datasets for both placement of CRL (without tearing) and mixing of PDP, the current evidence indicates that any potential asbestos exposures during these tasks would be low, if any exposure occurs at all. We, therefore, did not calculate cumulative exposure estimates for these scenarios. Further, because of a lack of quantitative exposure data, we did not characterize potential cumulative asbestos exposures associated with PDP bottle opening or after mixing.
Although dental hygienists and dental assistants were considered in the exposure assessment, they were not as likely to experience potential asbestos exposures from the use of asbestos-containing CRL as dentists and dental laboratory technicians, and therefore the cumulative exposure estimates presented in represent reasonable, upper-bound estimates for these professionals for this specific task; these two latter professions (dentists and dental laboratory technicians) likely worked more intimately with this product in laboratory and/or educational settings. Interestingly, Brune and Beltesbrekke (Citation1980) emphasized that a dental office, where dental hygienists and assistants typically work, “might be ‘cleaner’ with respect to air pollution compared to the laboratory” (Brune and Beltesbrekke Citation1980, p. 687). Conversely, PDP was likely only used in offices during surgeries. As such, dentists, dental hygienists, and dental assistants would have more likely been exposed to this product as opposed to dental laboratory technicians, if these professionals handled PDP at all.
As noted previously, for an individual working with asbestos-containing CRL (tearing, placement), upper-bound 8-h TWA estimates of 0.022 and 0.0044 f/cc were calculated based on airborne fiber concentrations measured for personal and area (bystander) exposures, respectively. These exposures were classified as Category 2 (well controlled) and Category 1 (highly controlled) for the personal and bystander scenarios, respectively, when the AIHA exposure profile categorization methodology was utilized. Further examination of novel exposure data associated with these products and tasks using Bayesian Decision Analysis in the context of our findings would be informative, particularly for the inclusion of any censored (i.e. non-detectable) data (Hewett et al. Citation2006; Jahn et al. Citation2015, Chapter 22, Appendix VIII).
In certain settings, it is possible that multiple workers manipulated CRL within the same room. Each individual worker therefore could have experienced both direct exposure to asbestos from their work and additional bystander exposure to asbestos from their colleagues’ work. However, it has been demonstrated that asbestos exposure concentrations decrease as distance from the source increases, and important variables that would likely influence airborne fiber concentrations (e.g. size of the laboratory space, ventilation, proximity to other workers, etc.) would have to be accounted for in characterizing any bystander exposure that might have occurred in these environments (Donovan et al. Citation2011).
Furthermore, maximum temperatures ranging from approximately 250–900 °C (approximately 500–1650 °F) are reached during the heat treatment step of the “lost-wax” casting method. Although asbestos is known for its resilience to heat, at high enough temperatures, asbestos fibers decompose and lose their physical and chemical properties (Dellisanti et al. Citation2001–2002). There are three steps in the thermal decomposition of asbestos minerals: loss of adsorbed water, removal of structural hydroxyl groups, and crystallization of amorphous materials after dehydroxylation (Kusiorowski et al. Citation2012). Both the reaction end-products and the temperature at which asbestos decomposes depend upon the type of asbestos being heated (Dellisanti et al. Citation2001–2002). For example, chrysotile is progressively converted to forsterite, silica, and eventually enstatite, beginning at 400 °C (approximately 750 °F) with complete conversion achieved at temperatures of ≥700 °C (approximately ≥1300 °F) (Dellisanti et al. Citation2001–2002; Virta Citation2002). The temperature range for the thermal decomposition of crocidolite is broad (approximately 400–900 °C, or 750–1650 °F) and creates iron and magnesium pyroxenes, magnetite, hematite, and silica, while asbestiform tremolite can withstand the highest temperatures (approximately 740–1040 °C, or 1400–1900 °F) before degradation occurs into pyroxenes (calcium, magnesium, and iron) and silica (Xu et al. Citation1996; Virta Citation2002). It has been demonstrated that fibrous morphology can persist after heating, but the fibers are fragile, and easily disintegrate into a powder end-product that is no longer carcinogenic, nor a concern for human health (Dellisanti et al. 2001-2002; Kusiorowski et al. Citation2012).
Potential presence of amphiboles in dental products
Chrysotile has generally been acknowledged as the only fiber type used in dental products in the U.S. (Dyer Citation1967; Bakdash and Frydman Citation1976; Davis Citation1986, Citation1987, Citation1995; Markowitz and Moline Citation2017). Bulk analyses ( and ) confirm that chrysotile was the predominant asbestos fiber type intentionally used in CRL and PDP. Indeed, it has been noted elsewhere that because of their flexible nature, chrysotile asbestos fibers, as opposed to amphibole asbestos fibers, which possess a more rigid structure, were specifically used in the manufacture of CRL for ease of manipulation and compressibility (Davis Citation1995).
Yet, according to at least one study, CRL may have contained trace amounts of tremolite/actinolite (Millette et al. Citation2019); the authors did not specify, however, if the fibers identified were of the asbestiform mineral habit. Understanding whether an amphibole mineral exists in the asbestiform versus non-asbestiform habit is crucial, as the non-asbestiform types of these minerals are not biologically active and are not regulated as “asbestos”, per se (Consumer Product Safety Commission [CPSC] Citation1988; American Thoracic Society [ATS] Citation1990; Occupational Safety and Health Administration [OSHA] Citation1992; Vu Citation1993; Agency for Toxic Substances and Disease Registry [ATSDR] Citation2001; Addison and McConnell Citation2008; Gamble and Gibbs Citation2008; Mossman Citation2008; Williams et al. Citation2013; Mossman Citation2018). The finding of trace amounts of amphibole minerals in CRL upon being ground up and digested is not surprising, as it has been reported that tremolite may be found as a contaminant in certain chrysotile deposits, and is therefore occasionally identified in chrysotile-containing products (Finley et al. Citation2012).
Additionally, Mensi et al. (Citation2017) reported one case of pleural mesothelioma in a dental laboratory technician who had an asbestos lung fiber burden of 22,000 fibers/g dry weight of lung tissue by SEM. A control population to which to compare lung fiber burden was not provided. The authors noted that “asbestos bodies are largely produced by long amphibole fibers, while production from chrysotile is negligible. Hence, we can hypothesize that chrysotile materials used by the patient were contaminated by amphibole asbestos” (Mensi et al. Citation2017, p. 447). Although potential asbestiform tremolite/actinolite exposures from the use of CRL were not calculated, these exposures, if any occurred at all, would most likely be negligible, based on the bulk content of CRL as reported by Millette et al. (Citation2019). These investigators did reportedly identify one “tremolite asbestos bundle” in a single air sample collected during CRL manipulation (Millette et al. Citation2019, p. 107). Based on the underlying report, however, this “bundle” was identified in the area sample collected (Compton and Millette Citation2010b). Yet, as noted, it appears that Millette et al. (Citation2019) only reported personal air sampling results. Further, it is not clear in what sample the “bundle of tremolite asbestos fibers”, which was reported in an additional unpublished report by Compton and Millette (Citation2010d), was found and this result was not reported in their published synopsis of these reports (Millette et al. Citation2019). Even if the evaluated airborne fiber exposures associated with CRL were comprised entirely of asbestiform tremolite, which would not be true given the available bulk and air sampling data summarized herein, the greatest personal cumulative exposure we calculated for this specific task (0.33 f/cc-years; ) is still less than the published asbestiform tremolite NOAEL for mesothelioma of 0.5–2.6 f/cc-years (Finley et al. Citation2012).
Furthermore, in their derivation of the chrysotile NOAEL for pleural mesothelioma, Pierce et al. (Citation2016) included various chrysotile-exposed cohorts who may have also been exposed to commercial amphiboles, including asbestiform tremolite, which would have the effect of biasing the NOAELs for mesothelioma toward the null. Similarly, the authors reported that “[t]he presence of other known or potential respiratory carcinogens, could also have biased the NOAELs for lung cancer toward lower values” (Pierce et al. Citation2016, p. 579). Additionally, in their calculation of the chrysotile NOAELs for ARD, Pierce et al. (Citation2016) included cohorts with reported exposure to long asbestos fibers; after stratifying for long versus short fiber length, the authors did not find any increased risk at any dose for short-fiber cohorts. In fact, the authors concluded, “to the best of our knowledge, there is no evidence indicating that historical users of short fiber chrysotile products are at increased risk of developing lung cancer or mesothelioma. In short, it may be inferred that processed short fiber chrysotile has very little, and perhaps no, carcinogenic potential” (Pierce et al. Citation2016, p. 577). In fact, Berman and Crump (Citation2003) prepared a report for the U.S. EPA in which they assessed asbestos-related risk and found the “possibility that pure chrysotile is non-potent for causing mesothelioma cannot be ruled out by the epidemiology data” (Berman and Crump Citation2003, p. 1.4). Since limited information exists in the available published literature regarding fiber length for the chrysotile used in dental products, further analytical studies may be warranted to elucidate any potential biological activity that these fibers may possess.
The first case of malignant pleural mesothelioma in a dental professional in the available literature was reported by Reid et al. (Citation1991). A 60-year-old retired dentist, with no other reported or apparent source of exposure to asbestos apart from asbestos-containing dental materials, had a lung burden of 0.7 × 106 crocidolite fibers/g of dry lung tissue; no comparison to lung fiber burden from a control population was made (Reid et al. Citation1991; Markowitz and Moline Citation2017). No chrysotile was identified, which the authors contributed to a clearance effect. However, Mensi et al. (Citation2017) pointed out that “[a]lthough biopersistence of amphibole fibers is greater than that of chrysotile fibers, clearance of long chrysotile fibers is slow or insignificant; therefore a ‘clearance effect’ does not seem to be an adequate explanation” (Mensi et al. Citation2017, p. 447). Reid and colleagues noted that “[t]his amount of crocidolite is not as high as would be expected in a case associated with longstanding occupational exposure, but represents a greater level of exposure than in the general population” (Reid et al. Citation1991, p. 696). They further reported that both crocidolite and chrysotile fibers were found in paper used in the manufacture of metal prostheses (fillings) and in powder used as a binder in periodontal dressing. Specifically, they noted that asbestos-containing paper was used to line casting rings and crucibles, and was supplied on large dry rolls “from which crocidolite fibers were readily released into the air” (Reid et al. Citation1991, p. 696). The authors do not cite their source for this information.
Although the case reported in Reid et al. (Citation1991) was likely British in nationality, Mensi et al. (Citation2017) stated that raw materials used in dental products in the U.S. were similar to those used in the U.K., suggesting that the dental products that were used by the Reid et al. (Citation1991) case likely only intentionally contained chrysotile. Further, Davis (Citation1995) reported that crocidolite is “expensive and not commonly used in dental applications”, such that the source of exposure in the Reid et al. (Citation1991) case “remains puzzling” (Davis Citation1995, p. 294). Other researchers have therefore postulated that the dentist described by Reid et al. (Citation1991), who was reported to have crocidolite in his lungs, may have had an alternative exposure to amphibole asbestos, such as from shipbuilding or from British gas masks manufactured and worn during World War II (Davis Citation1995; Mensi et al. Citation2017). To the best of our knowledge, the study performed by Reid et al. (Citation1991) is the only study in which crocidolite fibers were associated with asbestos-containing dental products. This finding, however, is not supported by any historical documents or analytical studies that were identified.
Weight of epidemiological evidence
Our findings from the risk assessment are generally consistent with the epidemiological literature in that available studies have not reported an increased risk of mesothelioma in dentists or other dental professionals. Similarly, no epidemiological evidence exists indicating that dental professionals are at an increased risk of lung cancer, likely due to decreased rates of smoking among this profession (Sankila et al. Citation1990; Rix and Lynge Citation1996; Nishio et al. Citation2004).
To date, we are aware of 12 cases of mesothelioma and one case of lung cancer described in the literature among dental professionals and their relatives who used and/or were bystanders to the use of potentially asbestos-containing dental products. However, in general, inferences of causation from case series and/or reports alone are highly subjective, which minimizes their validity and epidemiologic significance (Oliveira and Leles Citation2006; Nissen and Wynn Citation2014). Indeed, case series and reports are the least methodologically and statistically robust epidemiological tool, as these observational studies lack a control group comparison, ultimately hindering a causal assessment (Brownson and Petitti Citation2006; Oliveira and Leles Citation2006; Kempen Citation2011). As such, conclusions drawn from case series or reports are less reliable than those made from more robust epidemiological studies. The existence of some mesothelioma case reports scattered over a period of several decades among individuals who used CRL is likely not evidence of causation and can be explained simply by the background rate of mesothelioma in the general population, or possibly by an unknown or unidentified source (Price and Ware Citation2009; Moolgavkar et al. Citation2017).
The risk of nonmalignant respiratory disease (NMRD), including pneumoconiosis, pleural thickening, and pleural plaques, among dental professionals who work in mixed dust environments warrants further research. Many of the studies reviewed noted that the ventilation in the various dental laboratories studied was not adequate for mitigating dust exposures, with noise cited as a major hindrance to adopting proper ventilation (Brune et al. Citation1981; Rom et al. Citation1984; Lung disease in dental laboratory technicians Citation1985; Choudat et al. Citation1993; Jacobsen et al. Citation1996; Nayebzadeh et al. Citation1999; Kartaloglu et al. Citation2003). In fact, in one study, only 37.3% of dental laboratory technicians were found to have used ventilation during their work (Froudarakis et al. Citation1999). Dental laboratory technicians’ pneumoconiosis has therefore been suggested to be “a real entity differing from other pneumoconiosis [sic] such as silicosis, hard metal disease, or asbestosis” (Persson and Brune Citation1989, p. 339). Further, although pleural plaques have been commonly associated with prior exposure to asbestos, other risk factors for pleural plaque development, including empyema, tuberculosis, rib fractures, and hemothorax, have been identified (Clarke et al. Citation2006).
Limitations
The airborne fiber concentrations used in our exposure assessment were analyzed by PCM. Notably, this methodology is incapable of differentiating between asbestos and other fibers (National Institute for Occupational Safety and Health [NIOSH] Citation1994). As such, nearly all of the fiber data presented herein are not asbestos-specific. For the purposes of the current analysis, however, we conservatively assumed that all fibers identified were potentially asbestos, which likely led to an overestimation of any potential exposures associated with the use of the products evaluated herein.
We were further limited in our exposure assessment by the paucity of available measurable exposure data, specifically with regard to potential personal and area (bystander) exposures from the placement of CRL (without tearing), as well as during the mixing of PDP. The lack of measurable exposure data for these scenarios, however, is important to note because it most likely indicates that any potential asbestos exposure during these tasks would be very low, if measurable at all. Indeed, because no fibers were actually detected in any of these samples above the LOD during placement of CRL or mixing of PDP, actual exposures during these tasks may be as low as zero. The lack of data for PDP use specifically may be a result of the CDT/CDMD’s banning of this product in the mid-1970s (Council on Dental Therapeutics [CDT] 1976). In fact, in 1977, the International Agency for Research on Cancer (IARC) noted at that time that the use of PDP and CRL “may be a potential problem and has not been evaluated by adequate industrial hygiene monitoring” (International Agency for Research on Cancer [IARC] Citation1977, p. 36).
Notably, the LODs for two of the PDP mixing samples were above the current asbestos regulatory limit of 0.1 f/cc, expressed as an 8-h TWA, due to a limited volume of sampled air over sampling durations of <5 min. Thus, as a point of comparison and according to the methodology previously described, we calculated an upper-bound 8-h TWA of 0.0031 f/cc (= [0.297 f/cc × 5 min]/480 min) from the data describing mixing of PDP, assuming exposure at the LOD (typically, however, exposure scientists and others use substitution methods such as ½ of the LOD to analyze censored data (Jahn et al. Citation2015, Appendix VIII)). More realistically, an 8-h TWA of 0.00026 f/cc (= [0.0044 f/cc × 28 min]/480 min) was calculated for the longest duration sample, again assuming exposure at the LOD. These hypothetical (since no measurable fibers were actually reported for this task) 8-h TWA exposures would result in estimated personal cumulative exposures ranging from 0.0039 f/cc-years (=0.00026 f/cc × 15.1 years) to 0.047 f/cc-years (=0.0031 f/cc × 15.1 years) for dentists mixing PDP, which are still far below the relevant NOAEL benchmarks discussed above.
Furthermore, our analysis only included dental professionals, as these workers are thought to have the greatest potential for exposure to asbestos-containing dental products. The “lost-wax” casting method is also utilized to fabricate jewelry, aircraft engine parts, and metallic appliances used in orthopedic surgery (Hollenback Citation1962; Millette Citation2013). Indeed, a case of peritoneal mesothelioma was reported in a 49-year-old female silversmith who used asbestos-containing CRL as part of the “lost-wax” method to cast jewelry for one year in college (Markowitz and Moline Citation2017). As such, potential asbestos exposures for individuals in these professions using time-activity data specific to those various tasks could be evaluated in the future.
We would also like to note that we identified all but one of the underlying unpublished reports by MVA Scientific Consultants detailing their bulk analyses, glovebox studies, and chamber studies with CRL, which were summarized and synthesized by Millette et al. (Citation2019). We have included the reports we identified as Supplemental Material (Compton and Millette Citation2010a, Citation2010b, Citation2010c, Citation2010d, Citation2011a, Citation2011b, Citation2011c, Citation2011d, Citation2011e, Citation2012; Millette Citation2004, Citation2005a, Citation2005b, Citation2005c, Citation2005d, Citation2008). These initial analyses were mentioned by Millette et al. (Citation2019) in their Disclosure statement, though the authors did not formally cite any of these reports anywhere in their publication. Instead, Millette et al. (Citation2019) broadly described the methods and results of each category of study (i.e. bulk analyses vs. glovebox studies vs. chamber studies). Yet upon review of the underlying reports, we found that the methods used sometimes varied slightly between reports. For example, in the bulk analyses, SEM was only used in select reports (Millette Citation2008; Compton and Millette Citation2010a, Citation2010c) to analyze CRL particles potentially released on a finger following touching of the CRL material, while SEM with EDS was used in one report (Compton and Millette Citation2011e); TEM with EDS was only used in two reports (Compton and Millette Citation2011c, Citation2011e) to identify potential amphibole minerals. In addition, varying numbers of CRL tears were used in some chamber tests. Slight modifications in methods can hinder the interpretation of the resulting dataset when results are broadly grouped together.
Regarding our search of the NOMS PMR Query System, it is important to note that the database does not provide any information regarding potential use of asbestos-containing dental products. As such, we cannot be certain that any of the cases captured by this database actually used such products. Indeed, the database only aggregates mortality data from 1985 onward, which is on the cusp of when chrysotile was phased out of dental products altogether.
Additionally, although investment material did not intentionally contain asbestos since at least 1930 (if ever at all), this product does contain crystalline silica (Collard et al. Citation1991). A case of silicotuberculosis has been reported in a dental mechanic who polished dentures (Sweeney Citation1933; Slitzbach Citation1939; Earnshaw Citation1988). We did not attempt to evaluate any potential health risks associated with silica exposures apart from describing the literature regarding pneumoconiosis risk in dental professionals. Thus, more targeted future investigations of potential health risks associated with use of investment material may be warranted.
Conclusion
The weight of evidence demonstrates that upper-bound 8-h TWA personal airborne asbestos exposures, which were calculated based on measured airborne fiber concentrations, associated with tearing followed by placement of asbestos-containing CRL (0.022 f/cc) would not have exceeded the current or historical 8‐hour TWA OSHA PELs or ACGIH TLVs (0.1 f/cc or greater) for asbestos. Because all identified exposure data for mixing of PDP was non-measurable, 8-h TWAs were not calculated.
Furthermore, the greatest cumulative asbestos exposure for dental professionals associated with tearing followed by placement of CRL (0.33 f/cc-years) calculated in this study is well below published “best estimate” chrysotile NOAELs for lung cancer (89–168 f/cc-years) and pleural mesothelioma (208–415 f/cc-years). Similarly, the upper-bound calculated 8-h TWA concentration (0.0044 f/cc) and cumulative asbestos exposure estimate (0.066 f/cc-years) for tearing followed by placement of CRL derived from area (bystander) samples are also lower than current occupational exposure limits and guidelines for asbestos exposures, as well as the published chrysotile NOAELs for lung cancer and pleural mesothelioma, respectively. As such, dental professionals who historically used or were bystanders to the use of asbestos-containing CRL and/or PDP are not expected to have an increased risk of developing ARD, a finding that is consistent with the epidemiological literature describing disease risk among these professions.
| Abbreviations | ||
| ACGIH | = | American Conference of Governmental Industrial Hygienists |
| ACH | = | air changes per hour |
| ADA | = | American Dental Association |
| AHERA | = | Asbestos Hazard Emergency Response Act |
| AIHA | = | American Industrial Hygiene Association |
| ARD | = | asbestos-related disease |
| BAF | = | bronchoalveolar fluid |
| BLS | = | Bureau of Labor Statistics |
| CDC | = | Centers for Disease Control and Prevention |
| CDMD | = | Council on Dental Materials and Devices |
| CDT | = | Council on Dental Therapeutics |
| CFM | = | cubic feet per minute |
| CI | = | confidence interval |
| CRL | = | casting ring liner |
| EDS | = | energy-dispersive X-ray microanalysis |
| EDXA | = | energy-dispersive X-ray spectroscopy |
| LOD | = | limit of detection |
| NBS | = | National Bureau of Standards |
| NMRD | = | nonmalignant respiratory disease |
| NIOSH | = | National Institute for Occupational Safety and Health |
| NOAEL | = | no-observed-adverse-effect-level |
| NOMS | = | National Occupational Mortality Surveillance |
| OEL | = | occupational exposure limit |
| OR | = | odds ratio |
| OSHA | = | Occupational Safety and Health Administration |
| PAH | = | polycyclic aromatic hydrocarbons |
| PCM | = | phase contrast microscopy |
| PDP | = | periodontal dressing powder |
| PEL | = | permissible exposure limit |
| PLM | = | polarized light microscopy |
| PMR | = | proportionate mortality ratio |
| SEM | = | scanning electron microscopy |
| SIR | = | standardized incidence ratio |
| SMR | = | standardized mortality ratio |
| TEM | = | transmission electron microscopy |
| TLV | = | threshold limit value |
| TWA | = | time-weighted average |
| XRD | = | X-ray powder diffraction. |
Acknowledgements
The authors would like to thank and acknowledge their Cardno ChemRisk colleagues, Ms. Amanda Burns and Mr. Jason Lotter, for their critical review and feedback on the draft manuscript, as well as their former colleague, Ms. Grace Anderson, for her assistance with the initial research on this topic. The authors also extend their sincere appreciation to Ms. Carrie Kahn (Cardno ChemRisk) for her editorial assistance, as well as Mr. Michael Tyson (Cardno) for his graphic design assistance with the figures. The authors would like to extend a special note of appreciation to the four external reviewers who provided extensive comments on the original manuscript. The comments provided by reviewers, who were selected by the Editor and anonymous to the authors, helped improve the content and clarity of the revised manuscript.
Declaration of interest
All of the authors are employed by Cardno ChemRisk, a consulting firm that provides scientific advice to the government, corporations, law firms, and various scientific/professional organizations. Cardno ChemRisk has been engaged by numerous companies involved in asbestos litigation, and four of the authors (A. M. I., N. J., B. F., and S. G.) have been retained as experts in asbestos litigation; this analysis could be relied upon in future asbestos-related litigation. A small portion of the research associated with this work was originally done in preparation for litigation and was supported by a company that has been involved in asbestos-related litigation. However, the vast majority of time invested by the authors to prepare this paper was provided by their employer and no external funding was received specifically for the preparation of this manuscript. Furthermore, the work product, including the conclusions drawn, is exclusively those of the authors, and has not been influenced by anyone other than the authors. Aside from the authors and their colleagues listed in the Acknowledgments, no one, including clients of Cardno ChemRisk, has reviewed, commented on, or revised this paper prior to its initial submission for publication.
References
- Addison J, McConnell EE. 2008. A review of carcinogenicity studies of asbestos and non-asbestos tremolite and other amphiboles. Regul Toxicol Pharmacol. 52(1 Suppl):S187–S199.
- Agency for Toxic Substances and Disease Registry [ATSDR]. 2001. Toxicological profile for asbestos. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
- American Thoracic Society [ATS]. 1990. Health effects of tremolite. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, June 1990. Am Rev Resp Dis. 142(6 Pt 1):1453–1458.
- Anderson JN. 1956. Applied dental materials. Oxford: Blackwell Scientific Publishers.
- Asgarzadeh K, Mahler DB, Peyton FA. 1954. The behavior and measurement of hygroscopic expansion of dental casting investment. J Dent Res. 33(4):519–530.
- Attanoos RL, Churg A, Galateau-Salle F, Gibbs AR, Roggli VL. 2018. Malignant mesothelioma and its non-asbestos causes. Arch Pathol Lab Med. 142(6):753–760.
- Baghani Z, Kadkhodazadeh M. 2013. Periodontal dressing: a review article. J Dent Res Dent Clin Dent Prosp. 7(4):183–191.
- Bakdash MB, Frydman A. 1976. Asbestos in periodontal dressings: a possible health hazard. Quintessence Int Dent Dig. 7(10):61–65.
- Barrett TE, Pietra GG, Maycock RL, Rossman MD, Minda JM, Johns LW. 1989. Acrylic resin pneumoconiosis: report of a case in a dental student. Am Rev Respir Dis. 139(3):841–843.
- Berman DW, Crump KS. 2003. Final draft: technical support document for a protocol to assess asbestos-related risk (EPA# 9345.4-06). Washington (DC): U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response.
- Blair A, Walrath J, Rogot E. 1985. Mortality patterns among U.S. veterans by occupation. I. Cancer. J Natl Cancer Inst. 75(6):1039–1047.
- Bouchardy C, Schuler G, Minder C, Hotz P, Bousquet A, Levi F, Fisch T, Torhorst J, Raymond L. 2002. Cancer risk by occupation and socioeconomic group among men–a study by the Association of Swiss Cancer Registries. Scand J Work Environ Health. 28(Suppl 1):1–88.
- Brantley WA, Liu JC, Kos WL, Ziebert GJ. 1986. Evaluation of four substitutes for asbestos in lining casting rings. Oper Dent. 11(1):25–32.
- Brecker SC. 1961. Crowns; preparation of the teeth and construction of the various types of full coverage restorations. Philadelphia (PA): W. B. Saunders Co.
- Brennom EF. 1973. Technic for creating disposable inlay rings. Dent Surv. 49(4):40–41.
- Broomell IN. 1908. Practical dentistry, by practical dentists. Philadelphia (PA): L.D. Caulk Co.
- Brownson RC, Petitti DB. 2006. Applied epidemiology: theory to practice. 2nd ed. New York (NY): Oxford University Press.
- Brune D, Beltesbrekke H. 1980. Dust in dental laboratories. Part I: types and levels in specific operations. J Prosth Dent. 43(6):687–692.
- Brune D, Beltesbrekke H. 1981. Levels of methylmethacrylate, formaldehyde, and asbestos in dental workroom air. Scand J Dent Res. 89(1):113–116.
- Brune D, Beltesbrekke H, Melsom S. 1981. Dust in workroom air of dental laboratories. Swed Dent J. 5(5–6):247–251.
- Bureau of Labor Statistics [BLS]. 2019a. Occupational outlook handbook: dental and ophthalmic laboratory technicians and medical appliance technicians. Washington (DC): U.S. Department of Labor, Bureau of Labor Statistics [last modified 2019 Sep 4]. https://www.bls.gov/ooh/production/dental-and-ophthalmic-laboratory-technicians-and-medical-appliance-technicians.htm.
- Bureau of Labor Statistics [BLS] 2019b. Occupational Outlook Handbook: Dental Assistants. Washington (DC): U.S. Department of Labor, Bureau of Labor Statistics [last modified 2019 Oct 15]. https://www.bls.gov/ooh/healthcare/dental-assistants.htm.
- Bureau of Labor Statistics [BLS] 2019c. Occupational outlook handbook: dental hygienists. Washington (DC): U.S. Department of Labor, Bureau of Labor Statistics [last modified 2019 Dec 6]. https://www.bls.gov/ooh/healthcare/dental-hygienists.htm.
- Bureau of Labor Statistics [BLS]. 2019d. Occupational outlook handbook: dentists. Washington (DC): U.S. Department of Labor, Bureau of Labor Statistics [last modified 2019 Sep 4]. https://www.bls.gov/ooh/healthcare/dentists.htm.
- Burnett G. 1976. Letter to the editor: substitute for asbestos in casting rings. Br Dent J. 141(6):171.
- Choël L, Grosgogeat B, Bourgeois D, Descotes J. 1999. Occupational toxic risks in dental laboratory technicians. J Environ Med. 1(4):307–314.
- Choudat D. 1994. Occupational lung diseases among dental technicians. Tuber Lung Dis. 75(2):99–104.
- Choudat D, Triem S, Weill B, Vicrey C, Ameille J, Brochard P, Letourneux M, Rossignol C. 1993. Respiratory symptoms, lung function, and pneumoconiosis among self employed dental technicians. Br J Ind Med. 50(5):443–449.
- Clarke CC, Mowat FS, Kelsh MA, Roberts MA. 2006. Pleural plaques: a review of diagnostic issues and possible nonasbestos factors. Arch Environ Occup Health. 61(4):183–192.
- Coleman RL. 1928. Physical properties of dental materials (Gold alloys and accessory materials). Bur Stan J Res. 1(6):867–938.
- Collard SM, Vogel JJ, Ladd GD. 1991. Respirability, microstructure and filler content of composite dusts. Am J Dent. 4(3):143–151.
- Compton SP, Millette JR. 2010a. Report of results: MVA8587. Asbestos fiber analysis Kerr strip asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2010b. Report of results: MVA8587. Asbestos fiber release from a Kerr asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2010c. Report of results: MVA8589. Asbestos fiber analysis whip mix asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2010d. Report of results: MVA8589. Asbestos fiber release from a whip mix asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2011a. Report of results: MVA8589. Asbestos fiber analysis and release from a whip mix asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2011b. Report of results: MVA8589. Asbestos fiber release during tearing of a whip mix asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2011c. Report of results: MVA8814. Asbestos fiber analysis and release from a Kerr asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2011d. Report of results: MVA8814. Asbestos fiber release during tearing of a Kerr asbestos dental liner. Duluth (GA): MVA Scientific Consultants..
- Compton SP, Millette JR. 2011e. Report of results: MVA9045. Asbestos fiber analysis of asbestos dental liner. Duluth (GA): MVA Scientific Consultants.
- Compton SP, Millette JR. 2012. Report of results: MVA9046. Asbestos fiber analysis of dental materials – Petitpas case. Duluth (GA): MVA Scientific Consultants.
- Consumer Product Safety Commission [CPSC]. 1988. Briefing package of the CPSC Office of the Secretary on a petition to ban play sand with non-asbestiform tremolite. Washington (DC): U.S. Consumer Product Safety Commission.
- Cook A. 2008. Asbestos dressing. Br Dent J. 204(5):224.
- Council on Dental Therapeutics [CDT]. 1976. Hazards of asbestos in dentistry. J Am Dent Assoc. 92(4):777–778.
- Cutright DE, Huget EF, Brady JM. 1980. Asbestos: a subtle carcinogen in the dental laboratory. Gen Dent. 28(3):46–50.
- Davis DR. 1986. The nature and hazards of asbestos. Cda J. 14(6):28–34.
- Davis DR. 1987. Potential health hazards of ceramic ring lining material. J Prosth Dent. 57(3):362–369.
- Davis DR. 1995. Release of asbestos fibers during casting ring liner manipulation. J Prosthet Dent. 74(3):294–298.
- De Vuyst P, Vande Weyer R, De Coster A, Marchandise FX, Dumortier P, Ketelbant P, Jedwab J, Yernault JC. 1986. Dental technician's pneumoconiosis. A report of two cases. Am Rev Respir Dis. 133(2):316–320.
- Delgado VP, Peyton FA. 1953. The hygroscopic setting expansion of a dental casting investment. J Prosth Dent. 3(3):423–433.
- Dellisanti F, Minguzzi V, Morandi N. 2001–2002. Experimental results from thermal treatment of asbestos containing materials. GeoActa. 1:61–70.
- Dinger EJ. 1963. Guide to laboratory procedures for constructing a removable partial denture. Ann Arbor (MI): Overbeck.
- Donovan EP, Donovan BL, Sahmel J, Scott PK, Paustenbach DJ. 2011. Evaluation of bystander exposures to asbestos in occupational settings: A review of the literature and application of a simple eddy diffusion model. Crit Rev Toxicol. 41(1):50–72.
- Dyer MRY. 1967. The possible adverse effects of asbestos in gingivectomy pacts. Br Dent J. 122(11):507.
- Earnshaw R. 1988. The effect of casting ring liners on the potential expansion of a gypsum-bonded investment. J Dent Res. 67(11):1366–1370.
- Eriksson M, Hardell L, Malker H, Weiner J. 1998. Increased cancer incidence in physicians, dentists, and health care workers. Oncol Rep. 5(6):1413–1418.
- Finley BL, Pierce JS, Phelka AD, Adams RE, Paustenbach DJ, Thuett KA, Barlow CA. 2012. Evaluation of tremolite asbestos exposures associated with the use of commercial products. Crit Rev Toxicol. 42(2):119–146.
- Froudarakis ME, Voloudaki A, Bouros D, Drakonakis G, Hatzakis K, Siafakas NM. 1999. Pneumoconiosis among Cretan dental technicians. Respiration. 66(4):338–342.
- Fry C. 2008. Asbestos awareness. Br Dent J. 204(9):476–477.
- Fry C. 2009. An investigation into asbestos related disease in the dental industry. Br Dent J. 206(10):515–516.
- Fulton RS, Madden RM, Cheff SO. 1989. Ultrastructural pulmonary changes in mice exposed to aerosolized periodontal pack powder. Dent Mater. 5(3):194–200.
- Gamble J, Gibbs GW. 2008. An evaluation of the risks of lung cancer and mesothelioma from exposure to amphibole cleavage fragments. Regul Toxicol Pharmacol. 52(1 Suppl):S154–S186.
- Glass RL. 1966. Mortality of New England dentists 1921–1960, May, 1966. Public health service publication No.: 999-RH-18. Washington (DC): U.S. Department of Health, Education, and Welfare, Public Health Service, Division of Radiological Health.
- Guglani LM, Allen EF. 1965. Connective tissue reaction to implants of periodontal packs. J Periodontol. 36:279–282.
- Haldorsen T, Andersen A, Boffetta P. 2004. Smoking-adjusted incidence of lung cancer by occupation among Norwegian men. Cancer Causes Control. 15(2):139–147.
- Hewett P, Logan P, Mulhausen J, Ramachandran G, Banerjee S. 2006. Rating exposure control using Bayesian decision analysis. J Occup Environ Hyg. 3(10):568–581.
- Hill GB, Harvey W. 1972. The mortality of dentists. Br Dent J. 132(5):179–182.
- Hollenback GM. 1962. A brief history of the cast restoration. J S CA State Dent Assoc. 30(1):8–18.
- Hollenback GM. 1964. Chapter 1: history of the cast restoration science and technic of the cast restoration. St. Louis (MO): C.V. Mosby; p. 17–33.
- Hollenback GM, Rhoads JE. 1960. A comparison of the linear expansion of investment with the linear casting shrinkage of gold: part IV. J S CA State Dent Assoc. 28(2):40–46.
- Infante PF, Lemen RA. 1976. Letter: asbestos in dentistry. J Am Dent Assoc. 93(2):221–222.
- International Agency for Research on Cancer [IARC]. 1977. Asbestos. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 14. Lyon: World Health Organization, International Agency for Research on Cancer; p. 11–81.
- J. F. Jelenko & Co. 1963. Crown and bridge construction: a handbook of dental laboratory procedures. 5th ed. New Rochelle (NY): Jelenko.
- Jacobsen N, Derand T, Hensten-Pettersen A. 1996. Profile of work-related health complaints among Swedish dental laboratory technicians. Commun Dent Oral Epidemiol. 24(2):138–144.
- Jahn SD, Bullock WH, Ignacio JS. 2015. A strategy for assessing and managing occupational exposures. 4th ed. Fairfax (VA): American Industrial Hygiene Association (AIHA).
- Jung IH. 2011. Airborne asbestos concentrations of dental laboratories in one metropolitan city. J Korea Conv Soc. 2:31–36.
- Kartaloglu Z, Ilvan A, Aydilek R, Cerrahoglu K, Tahaoglu K, Baloglu H, Misirli Z. 2003. Dental technician's pneumoconiosis: mineralogical analysis of two cases. Yonsei Med J. 44(1):169–173.
- Kempen JH. 2011. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol. 151(1):7–10.e1.
- Koifman S, Malhao TA, Pinto de Oliveira G, de Magalhaes Camara V, Koifman RJ, Meyer A. 2014. Cancer mortality among Brazilian dentists. Am J Ind Med. 57(11):1255–1264.
- Kusiorowski R, Zaremba T, Piotrowski J, Adamek J. 2012. Thermal decomposition of different types of asbestos. J Therm Anal Calorim. 109(2):693–704.
- Lacy AM, Fukui H, Jendresen MD. 1983. Three factors affecting investment setting expansion and casting size. J Prosthet Dent. 49(1):52–58.
- Legan J, Madden R, Thoma G, Patten J. 1973. Biologic exposure to dental materials. Oral Surg Oral Med Oral Pathol. 36(6):908–914.
- Loewen GM, Weiner D, McMahan J. 1988. Pneumoconiosis in an elderly dentist. Chest. 93(6):1312–1313.
- Lung disease in dental laboratory technicians. 1985. Lancet. 1(8439):1200–1201.
- Maguire SR. 1993. Employer and occupational tenure: 1991 update. Mon Labor Rev. 116(6):45–60.
- Markowitz SB, Moline JM. 2017. Malignant mesothelioma due to asbestos exposure in dental tape. Am J Ind Med. 60(5):437–442.
- Menck HR, Henderson BE. 1976. Occupational differences in rates of lung cancer. J Occup Med. 18(12):797–801.
- Mensi C, Ciullo F, Barbieri GP, Riboldi L, Somigliana A, Rasperini G, Pesatori AC, Consonni D. 2017. Pleural malignant mesothelioma in dental laboratory technicians: a case series. Am J Ind Med. 60(5):443–448.
- Millette JR. 2004. Report of results MVA6171. Asbestos fiber release dental casting ring liner. Duluth (GA): MVA Scientific Consultants.
- Millette JR. 2005a. Report of results MVA6352. Asbestos fiber examination dental casting ring liner. Duluth (GA): MVA Scientific Consultants.
- Millette JR. 2005b. Report of results MVA6352. Asbestos fiber release dental casting ring liner. Duluth (GA): MVA Scientific Consultants.
- Millette JR. 2005c. Report of results: MVA6353. Asbestos fiber release dental casting ring liner PK-1. Duluth (GA): MVA Scientific Consultants.
- Millette JR. 2005d. Report of results: MVA6353. Asbestos fiber release dental casting ring liner PK-2. Duluth (GA): MVA Scientific Consultants.
- Millette JR. 2008. Report of results: MVA7382. Asbestos fiber analysis dental casting ring liner. Duluth (GA): MVA Scientific Consultants.
- Millette JR. 2013. Supplemental report of results: MVA9389. Examination of jewelry making products for amphibole asbestos – Paul Greene case. Duluth (GA): MVA Scientific Consultants.
- Millette JR, Compton S, DePasquale C. 2019. Microscopical analyses of asbestos-containing dental tape. Microscope. 67(3):99–109.
- Moolgavkar SH, Chang ET, Mezei G, Mowat FS. 2017. Chapter 3, epidemiology of mesothelioma. In: Testa JR, editor. Asbestos and mesothelioma current cancer research. Cham, Switzerland: Springer; p. 43–72.
- Morey EF. 1991a. Dimensional accuracy of small gold alloy castings. Part 1. A brief history and the behaviour of inlay waxes. Aust Dent J. 36(4):302–309.
- Morey EF. 1991b. Dimensional accuracy of small gold alloy castings. Part 2. Gold alloy shrinkage. Aust Dent J. 36(5):391–396.
- Morey EF. 1992a. Dimensional accuracy of small gold alloy castings. Part 3. Gypsum-bonded investment expansion. Aust Dent J. 37(1):43–54.
- Morey EF. 1992b. Dimensional accuracy of small gold alloy castings. Part 4. The casting ring and ring liners. Aust Dent J. 37(2):91–97.
- Morey EF, Earnshaw R. 1992. The fit of gold-alloy full-crown castings made with pre-wetted casting ring liners. J Dent Res. 71(12):1858–1864.
- Morgenroth K, Kronenberger H, Michalke G, Schnabel R. 1985. Morphology and pathogenesis of pneumoconiosis in dental technicians. Pathol Res Pract. 179(4-5):528–536.
- Mossman BT. 2008. Assessment of the pathogenic potential of asbestiform vs. nonasbestiform particulates (cleavage fragments) in in vitro (cell or organ culture) models and bioassays. Regul Toxicol Pharmacol. 52(1 Suppl):S200–S203.
- Mossman BT. 2018. Mechanistic in vitro studies: What they have told us about carcinogenic properties of elongated mineral particles (EMPs). Toxicol Appl Pharmacol. 361:62–67.
- Moyer RS. 1959. A laboratory procedure guide for operative and crown bridge technics. Ann Arbor (MI): Overbeck.
- National Institute for Occupational Safety and Health [NIOSH]. 1994. NIOSH method 7400: asbestos and other fibers by phase contrast microscopy. NIOSH manual of analytical methods. Cincinnati, OH: National Institute for Occupational Safety and Health.
- Nayebzadeh A, Dufresne A, Harvie S, Begin R. 1999. Mineralogy of lung tissue in dental laboratory technicians' pneumoconiosis. Am Ind Hyg Assoc J. 60(3):349–353.
- Naylor WP, Moore BK, Phillips RW. 1987. A topographical assessment of casting ring liners using scanning electron microscopy (SEM). Quint Dent Tech. 11(6):415–420.
- Nishio N, Tanaka H, Tsukuma H, Tokunaga R. 2004. Lung cancer risk in male dentists: a retrospective cohort study in Japan, 1964–1997. J Occup Health. 46(1):37–42.
- Nissen T, Wynn R. 2014. The clinical case report: a review of its merits and limitations. BMC Res Notes. 7:264.
- Occupational Safety and Health Administration [OSHA]. 1992. 29 CFR parts 1910 and 1926: occupational exposure to asbestos, tremolite, anthophyllite and actinolite; final rule. Fed Reg. 57(110):24310–24331.
- Ogus H, Bourne R. 2007. Obituary: Richard James Bourne. Br Dent J. 203 (12):715.
- Ohno H, Miyakawa O, Shiokawa N. 1982. Distortion of a MOD pattern caused by the setting and hygroscopic expansion of the investment in the casting ring. II. Influence of W/P ratio, pattern position in the ring, and the condition of the asbestos lining. Dent Mater J. 1(1):47–54.
- Oliveira GJ, Leles CR. 2006. Critical appraisal and positive outcome bias in case reports published in Brazilian dental journals. J Dent Educ. 70(8):869–874.
- Otterson EJ, Arra MC. 1974. Potential hazards of asbestos in periodontal packs. J Wisc Dent Assoc. 50(11):435–438.
- Palmen N. 2005. Criteria document for Swedish occupational standards: cobalt and cobalt compounds. Vol. 2005. Stockholm: Arbetslivsinstitutet. (Arbete och hälsa); p. 12.
- Persson B, Brune D. 1989. Chapter 18: dental laboratories. In: Brune DK, Edling C, editors. Occupational hazards in the health professions. Boca Raton (FL): CRC Press; p. 333–345.
- Pesch B, Kendzia B, Gustavsson P, Jockel K, Johnen G, Pohlabeln H, Olsson A, Ahrens W, Gross IM, Bruske I, et al. 2012. Cigarette smoking and lung cancer-relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 131(5):1210–1219.
- Pierce JS, McKinley MA, Paustenbach DJ, Finley BL. 2008. An evaluation of reported no-effect chrysotile asbestos exposures for lung cancer and mesothelioma. Crit Rev Toxicol. 38(3):191–214.
- Pierce JS, Ruestow PS, Finley BL. 2016. An updated evaluation of reported no-observed adverse effect levels for chrysotile asbestos for lung cancer and mesothelioma. Crit Rev Toxicol. 46(7):561–586.
- Price B, Ware A. 2009. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 39(7):576–588.
- Priest G, Horner JA. 1980. Fibrous ceramic aluminum silicate as an alternative to asbestos liners. J Prosth Dent. 44(1):51–56.
- Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparen P, Tryggvadottir L, Weiderpass E, Kjaerheim K. 2009. Occupation and cancer – follow-up of 15 million people in five Nordic countries. Acta Oncol. 48(5):646–790.
- Radi S, Dalphin JC, Manzoni P, Pernet D, Leboube MP, Viel JF. 2002. Respiratory morbidity in a population of French dental technicians. Occup Environ Med. 59(6):398–404.
- Reid AS, Causton BE, Jones JS, Ellis IO. 1991. Malignant mesothelioma after exposure to asbestos in dental practice. Lancet. 338(8768):696.
- Rix BA, Lynge E. 1996. Cancer incidence in Danish health care workers. Scand J Soc Med. 24(2):114–120.
- Rom WN, Lockey JE, Lee JS, Kimball AC, Bang KM, Leaman H, Johns RE, Jr., Perrota D, Gibbons HL. 1984. Pneumoconiosis and exposures of dental laboratory technicians. Am J Public Health. 74(11):1252–1257.
- Sankila R, Karjalainen S, Laara E, Pukkala E, Teppo L. 1990. Cancer risk among health care personnel in Finland, 1971-1980. Scand J Work Environ Health. 16(4):252–257.
- Selden AI, Persson B, Bornberger-Dankvardt SI, Winstrom LE, Bodin LS. 1995. Exposure to cobalt chromium dust and lung disorders in dental technicians. Thorax. 50(7):769–772.
- Setcos JC, Vrijhoef MM, Blumershine R, Phillips RW. 1983. Airborne particles from alginate powders. J Am Dent Assoc. 106(3):355–358.
- Shell JS. 1968a. Setting and thermal expansion of investments. I. Effect of short vs. full length asbestos liners. J Ala Dent Assoc. 52(3):17–21.
- Shell JS. 1968b. Setting and thermal expansion of investments. II. Effects of: changes in water/powder ratio, dry vs. wet liners, and liner short on one end of ring only. J Ala Dent Assoc. 52(4):22–25.
- Shell JS. 1969. Setting and thermal expansion of investments. 3. Effects of: no asbestos liner, coating asbestos with petroleum jelly, and double asbestos liner. J Ala Dent Assoc. 53(1):31–34.
- Sherson D, Maltbaek N, Heydorn K. 1990. A dental technician with pulmonary fibrosis: a case of chromium-cobalt alloy pneumoconiosis? Eur Respir J. 3(10):1227–1229.
- Sherson D, Maltbaek N, Olsen O. 1988. Small opacities among dental laboratory technicians in Copenhagen. Br J Ind Med. 45(5):320–324.
- Shibuya M, Ohsawa M, Matsumoto H, Hisatsune K, Yasuda K. 1997. Anisotropic expansion in gypsum-bonded cristobalite investment mold. Dent Mater J. 16(1):48–59.
- Shimpo H, Yokoyama E, Tsurumaki K. 1998. Causes of death and life expectancies among dentists. Int Dent J. 48(6):563–570.
- Sichletidis L, Spyratos D, Chloros D, Michailidis K, Fourkiotou I. 2009. Pleural plaques in dentists from occupational asbestos exposure: a report of three cases. Am J Ind Med. 52(12):926–930.
- Simning A, van Wijngaarden E. 2007. Literature review of cancer mortality and incidence among dentists. Occup Environ Med. 64(7):432–438.
- Skinner EW. 1963. Some recent technical advance in dental materials. J Am Dent Assoc. 66:176–182.
- Slitzbach LE. 1939. The silicosis hazard in mechanical dentistry. JAMA. 113(12):1116–1119.
- Solomon ES. 2012. The past and future evolution of the dental workforce team. J Dent Ed. 76(8):1028–1035.
- Souder WH, Paffenbarger GC. 1942. Physical properties of dental materials. United States National Bureau of Standards Circular C433. Washington (DC): U.S. Government Publishing Office.
- Souder W, Sweeney WT. 1930. Tentative specifications for dental casting investment. J Am Dent Assoc. 17(5):780–782.
- Swartz ML. 1969. Research in dental materials. J Am Dent Assoc. 79(4):901–917.
- Sweeney WT. 1933. Cristobalite for dental investment. J Am Dent Assoc. 20(1):108–119.
- Taggart WH. 1907. A new and accurate method of making gold inlays. Dent Cosmos. 49(11):1117–1121.
- Takahashi J, Okazaki M, Kimura H, Hiraiwa K, Iwakawa Y, Mori S, Joshin K, Moriwaki Y, Doi Y. Y. 1983. The accuracy of occlusal cusp height in cast crowns. J Prosth Dent. 50(3):392–398.
- Taylor NO, Paffenbarger GC, Sweeney WT. 1930. Dental inlay casting investments: physical properties and a specification. J Am Dent Assoc. 17(12):2266–2286.
- Torbica N, Krstev S. 2006. World at work: dental laboratory technicians. Occup Environ Med. 63(2):145–148.
- Tuengerthal S, Kronenberger H, Meyer-Sydow J, Morgenroth H, Rieman H, Schneider M. 1983. Radiological findings in chest x-ray examinations of dental technicians. Proceedings of the Sixth International Pneumoconiosis Conference; Geneva: ILO. p. 1201–1210.
- Tulachka GJ, Brantley WA, McGivney GP, Ziebert GJ. 1987. An investigation of the potential biohazards associated with asbestos-substitute casting ring liners. J Prosthet Dent. 57(1):108–112.
- Virta RL. 2002. Asbestos: geology, mineralogy, mining, and uses (open-file report 02-149). Reston (VA): U.S. Department of the Interior, U.S. Geological Survey.
- Vu VT. 1993. Chapter 19, regulatory approaches to reduce human health risks associated with exposures to mineral fibers. In: Guthrie Jr. GD, Mossman BT, editors. Health effects of mineral dusts Volume 28: Reviews in Mineralogy. Washington (DC): Mineralogical Society of America; p. 545–554.
- Wagner M. 1985. Safeguarding the physical well-being of dentists. J Am Dent Assoc. 110(1):16–24.
- Williams C, Dell L, Adams R, Rose T, Van Orden D. 2013. State-of-the-science assessment of non-asbestos amphibole exposure: Is there a cancer risk? Environ Geochem Health. 35(3):357–377.
- Woody RD, Huget EF, Cutright DE. 1977. Characterization of airborne particles from irreversible hydrocolloids. J Am Dent Assoc. 94(3):501–504.
- Xu H, Veblen DR, Luo G, Xue J. 1996. Transmission electron microscopy study of the thermal decomposition of tremolite into clinopyroxene. Am Min. 81:1126–1132.
- Yewe-Dyer M. 2008. Carcinogenic asbestos. Br Dent J. 204(10):544.
- Yli-Urpo A, Oilo G, Syverud M. 1982. The effect of asbestos-alternatives on the accuracy of cast veneer crowns. Swed Dent J. 6(3):127–131.