ABSTRACT
Objective
To investigate the incidence and risk factors of small-for-gestational age (SGA) short stature at 2 and 3 years of age in SGA offspring born to women with hypertensive disorders of pregnancy (HDP).
Methods
We examined 226 women with HDP whose respective SGA offspring were delivered.
Results
Eighty offspring (41.2%) were diagnosed with SGA short stature. The prematurity before 32 weeks of gestation was the most significant factor for catch-up growth failure.
Conclusion
In SGA offspring born to women with HDP, SGA short stature incidence was high, and the risk factor was prematurity before 32 weeks of gestation.
Introduction
Hypertensive disorders of pregnancy (HDP) occur in approximately 5% of all pregnancies (Citation1). The etiology of HDP, particularly preeclampsia, is clarified by the two-stage disorder theory (Citation2,Citation3), wherein stage 1 involves placental hypoxia because of poor arterial perfusion, and stage 2 involves the subsequent release of hypoxia-induced factors that results in a disordered state, such as preeclampsia (Citation2–4). Anti-angiogenic factors released due to hypoxia further exacerbate the hypoxic placental insufficiency (Citation5,Citation6), causing hypertension and proteinuria in the mother (Citation7) and fetal growth restriction (FGR) and small-for-gestational age (SGA) in the fetus (Citation8).
Appropriate-for-gestational age neonates with birth weights and lengths below the 10th percentile are diagnosed as SGA (Citation9). Approximately 10% of SGA offspring cannot achieve catch-up growth and attain a length of ≥ −2.0 standard deviations (SD) by 2 years of age. Thus, they are diagnosed with short stature in children born SGA (Citation10,Citation11), which reportedly accounts for approximately 20% of cases of adulthood short stature (Citation12). A better understanding of HDP and its association with SGA prevalence can guide preventative and early interventional methodologies to greatly improve the life-course development of SGA short stature neonates.
In the general obstetric population, the reported risk factors for short stature in children born SGA include the birth length and a preterm birth at <32 weeks (Citation13). Although HDP causes premature delivery at a very early gestational age and severe FGR owing to impaired blood flow during the fetal period associated with placental circulatory failure (Citation8), reports on the incidence of and risk factors for short stature in children born SGA and failure of catch-up growth in SGA offspring born to women with HDP are lacking.
Therefore, we aimed to examine the incidence and risk factors for short stature in children born SGA and failure of catch-up growth in SGA offspring born to women with HDP.
Materials and methods
Participants, groups, and data collection
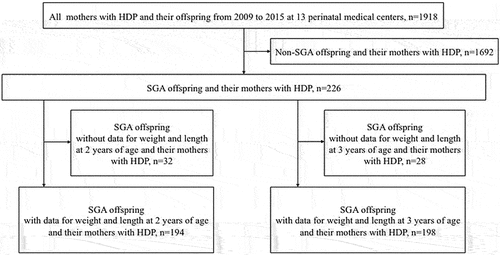
We retrospectively examined 1918 women with HDP who gave birth between 2009 and 2015 at 13 perinatal medical centers in eight prefectures in Chugoku and Shikoku, Japan. HDP was diagnosed according to the definition of the Japan Society for the Study of Hypertension in Pregnancy (Citation14). HDP is defined as hypertension (blood pressure≥140/90 mmHg) occurring during pregnancy and includes chronic hypertension, gestational hypertension, preeclampsia, and superimposed preeclampsia. Mothers included in this study had preeclampsia or superimposed preeclampsia. Preeclampsia is defined as gestational hypertension accompanied by one or more of the following new-onset conditions at or after 20 weeks gestation. These conditions could be proteinuria or other maternal organ dysfunctions, including liver involvement, progressive kidney injury, neurological complications, hematological complications, or uteroplacental dysfunction. However, all symptoms are normalized by 12 weeks postpartum. Superimposed preeclampsia is defined as hypertension that is diagnosed pre-pregnancy or before 20 weeks of gestation and followed by new onset conditions, as in preeclampsia at or after 20 weeks of gestation. A total of 226 SGA offspring (males, 115; females, 111) and their women with HDP were included in this study ().
The SGA offspring included in this study had both birth weights and lengths below the 10th percentile and either birth weight or length of < −2.0 SD (weight data alone were used if no length data were available). The SGA definition by the Japanese Society for Pediatrics Endocrinology was adopted (Citation15). This study excluded multiple pregnancies and pregnancies with chromosomal abnormalities and congenital disease in the fetuses and newborns to eliminate other conditions causing FGR. All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2018. This study was conducted with the approval of the Ethics Committees of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (Okayama, Japan). The information used herein, including the research plans, has been previously published online. All data were anonymized before the analysis. The requirement for informed consent was waived due to the retrospective nature of the study.
First, 194 SGA offspring (males, 102; females, 92) with data for the weight and length at 2 years of age were divided into two groups: those with a length of < −2.0 SD at 2 years of age were assigned to the SGA short stature group, and those with a length of ≥ −2.0 SD at 2 years of age were assigned to the non-SGA short stature group (). We examined the incidence of short stature in children born SGA at 2 years of age among SGA offspring born to women with HDP. The initial total of 226 participants decreased because we included only SGA offspring with data at 2 years of age. We compared the maternal and neonatal characteristics of the aforementioned groups. The maternal characteristics were the maternal age, height, type of pregnancy, type of HDP, gestational day of onset of HDP, day of delivery, days from onset of HDP to delivery, and method of delivery. The neonatal characteristics were the birth weight, birth length, birth weight SD, birth length SD, Apgar score at 1 min, Apgar score at 5 min, and neonatal complications; respiratory distress syndrome, chronic lung disease, sepsis, intraventricular hemorrhage, and necrotic enteritis. Breastfeeding, artificial milk feeding, and mixed nutrition during the first 6 months after birth were also compared. Additionally, to identify the factors that most significantly contributed to short stature in children born SGA at 2 years of age, we compared the maternal age, height, type of pregnancy, type of HDP, week of delivery (≥32 vs.<32 weeks), and offspring sex between the groups using multivariate analysis.
Next, 198 SGA offspring (males, 104; females, 94) with data for the weight and length at 3 years of age were divided into two groups: those with a length of < −2.0 SD at 3 years of age were assigned to the non-catch-up group, and those with a length of ≥ −2.0 SD at 3 years of age were assigned to the catch-up group (). The initial total of 226 participants decreased because we included only SGA offspring with data at 3 years of age. We also compared the maternal age, height, type of pregnancy, type of HDP, week of delivery (≥32 vs.<32 weeks), and offspring sex between the groups to identify the factors that most significantly contributed to the failure of catch-up growth at 3 years of age using multivariate analysis.
Statistical analyses
For statistical analyses, the Mann-Whitney U and χ2 tests were performed to determine statistical differences between the groups (first: the SGA short stature group and the non-SGA short stature group, second: the non-catch-up group and the catch-up group) using GraphPad Prism, version 8.0 (Graph Pad Software Inc., Los Angeles). Multivariate analysis using binomial logistic regression was performed using SPSS software, version 25.0 (SPSS Japan, Tokyo, Japan). A P-value of<0.05 was considered statistically significant.
Results
First, of the 194 SGA offspring for whom 2-year data were available, the incidence of short stature in children born SGA was 41.2% among SGA offspring born to women with HDP. Regarding the comparisons of maternal characteristics between the SGA short stature group and the non-SGA short stature group, significant differences were observed in height (P = 0.01), types of HDP (P = 0.03), gestational day of onset of HDP (P < 0.001), and day of delivery (P < 0.001). The commonest gestational age at delivery was at 27–31 weeks in the SGA short stature group and 32–36 weeks in the non-SGA short stature group ().
Table 1. Comparison of maternal characteristics between the SGA and non-SGA short stature groups.
Regarding the comparisons of neonatal characteristics between the SGA short stature group and the non-SGA short stature group, significant differences were observed in the birth weight (P < 0.001), birth length (P < 0.001), birth weight SD (P < 0.001), birth length SD (P = 0.003), Apgar score at 1 min (P < 0.001), Apgar score at 5 min (P = 0.001), and chronic lung disease (P < 0.001) (). However, the nutritional status during the first 6 months after birth did not significantly differ (data not shown).
Table 2. Comparison of neonatal characteristics between the SGA and non-SGA short stature groups.
In multivariate analysis, significant differences were found in the maternal height (0.87–0.98; odds ratio [OR], 0.93; 95% confidence interval [CI]) and day of delivery (OR, 3.93; 95% CI, 2.09–7.40;) ().
Table 3. Comparison of the maternal age, height, types of pregnancy, types of HDP, gestational age, and sex of the offspring between the SGA and non-SGA short stature groups by logistics regression analysis.
Next, of the 198 SGA offspring for whom 3-year data were available, 72 (36.4%) were assigned to the non-catch-up group and 126 (63.6%) to the catch-up group. The multivariate analysis revealed a significant difference in the day of delivery (OR, 2.75; 95% CI, 1.43–5.26;) and a tendency of difference in the maternal height (OR, 0.95; 95% CI, 0.89–1.00;) and offspring sex (OR, 1.85; 95% CI, 0.99–3.45;) ().
Table 4. Comparison of the maternal age, height, types of pregnancy, types of HDP, day of delivery, and sex of the offspring between the non-catch-up and catch-up groups by logistics regression analysis.
Discussion
Reportedly, approximately 90% of SGA children achieve catch-up growth after birth and are expected to reach normal height within 2 years of age (Citation12,Citation16). However, in this study, 80 (41.2%) of the SGA offspring born to women with HDP could not catch-up within 2 years of age and were diagnosed with short stature in children born SGA. The incidence of short stature in SGA offspring born to women with HDP was higher than in previous reports. In a 3-year prospective study of 604 preterm offspring born before 32 weeks of gestation, 27 (36.5%) of 74 offspring diagnosed with SGA at birth developed short stature in children born SGA at the age of 2 years, and 14 (18.9%) were targeted for treatment with growth hormone (Citation17). Engstrom et al. reported a positive correlation between the insulin-like growth factor 1 (IGF-1) concentration and postnatal growth (Citation18). The IGF-1 concentration increased at 30 weeks of gestational age (Citation19), although premature offspring born before 32 weeks of gestation had low plasma IGF-1 levels in mid-childhood (Citation20). These reports suggest that a short gestation increases the risk of short stature after birth. Here, the commonest gestational age at delivery was at 27–31 weeks in the SGA short stature group and 32–36 weeks in the non-SGA short stature group. Additionally, according to the multivariate analysis, the most influential factor for the high incidence of short stature in children born SGA and failure of catch-up growth within 3 years of age was the gestational age. Thus, it is suggested that the most important factor affecting the incidence of short stature in children born SGA and failure of catch-up growth among SGA offspring born to women with HDP is the gestational age. Such offspring are associated with severe intrauterine growth restriction and preterm birth and are at a high risk of short stature in children born SGA and failure of catch-up growth within 3 years of age.
However, it is well established that low-birth-weight infants are more likely to develop non-communicable diseases (NCDs), including cardiovascular diseases, later in life (Citation21). SGA and preterm infants are at a higher risk of NCDs if they exhibit rapid catch-up growth (Citation22). It is important to note that both rapid catch-up growth and overnutrition in low-birth-weight infants could lead to NCDs (Citation23).
There is a correlation between the maternal and newborn physique, and offspring born to short-statured women often become SGA (Citation24,Citation25). It has been reported that the risk factors for failure of catch-up growth in SGA offspring born at >32 weeks of gestation are a shorter maternal height and smaller fetal head circumference at birth (Citation14). However, another report concluded that the outcome of SGA offspring is not necessarily correlated with maternal height (Citation26). It has also been reported that the final height of preterm offspring is less affected by genetic factors and that the height at 2 years of age is important (Citation27). In this study, maternal height contributed to the catch-up growth at 3 years of age in SGA offspring born to women with HDP. Additionally, one report described strong associations between maternal short stature and severe types of preeclampsia (Citation28). Maternal height influences the physical development of SGA offspring through the onset and severity of HDP, suggesting that maternal short stature is a risk factor for intrauterine and extrauterine growth restrictions.
As previously stated, it is difficult for pregnant women who develop HDP to extend the gestational period after the onset, and many of them deliver prematurely. In this study, many women delivered at <32 weeks of gestation, and it has been reported that this factor tends to hinder postnatal growth. Here, several offspring did not achieve catch-up growth at the ages of 2 and 3 years, despite the gestational age being close to term and their physique at birth being almost normal. No differences were recorded among these cases regarding maternal and neonatal characteristics. Thus, along with the gestational age and physique at birth, postnatal environmental factors (living environment, post-weaning nutrition, and others) may be involved in the growth of the offspring.
In this study, sex differences were observed between the catch-up and non-catch-up groups at 3 years of age. We found that the male sex was a risk factor for catch-up growth failure at 3 years of age. Reese et al. showed that the independent factors contributing to the incidence of extrauterine dysgenesis are male sex, the need for 1-day-old assisted ventilation, a history of necrotizing enterocolitis, the need for respiratory assistance at 28 days of age, and in-hospital administration of steroids (Citation29). Moreover, it was reported that the female offspring with FGR born to women with HDP caught up faster than the male offspring, suggesting the presence of a sex difference in the risk of future obesity in offspring born to women with HDP (Citation30). Vatten et al. reported that the female offspring born to women with preeclampsia had a significantly higher body weight, body mass index, and blood pressure at 13–19 years of age than those born to women without preeclampsia (Citation31). It was also reported that although the female offspring born to women with preeclampsia had a higher body mass index and larger waist circumference than those born to women with normal pregnancies, no difference was recorded in the male offspring (Citation32). Therefore, male sex at birth from women with HDP may be a risk factor for failure of catch-up growth, while female sex at birth from women with HDP may be a risk factor for obesity and hypertension in adulthood.
This is the first report focusing on catch-up growth in SGA offspring born to HDP women. However, this study had limitations that should be acknowledged. This was a retrospective study conducted at multiple perinatal medical centers. Thus, there is a risk of bias inherent to the retrospective design that could affect the generalizability of the findings to other populations. In addition, the 13 participating perinatal medical centers in this study were limited to those located in the Chugoku-Shikoku region in Japan; therefore, the environmental factors were not examined. Furthermore, we only measured the mothers’ height and not the fathers.’ Our study was also limited to studies conducted among children up to the age of 3 years. Future studies should monitor the growth of offspring until adolescence and collect information on environmental factors and lifestyle choices; this would help account for potential confounding factors in such a longitudinal study.
In conclusion, the incidence of short stature in SGA offspring born to mothers with HDP was high (41.2%) in our study. SGA offspring born to women with HDP are at risk of early preterm birth, suggesting a high risk of short stature in children born SGA and catch-up growth failure within 3 years of age. In SGA offspring born to women with HDP, the gestational age was the most important factor affecting the incidence of short stature in children born SGA and failure of catch-up growth. Our findings present an opportunity to develop guidance for specialized care for women at risk who are pregnant or planning for pregnancy.
Consent to participate
The requirement for informed consent was waived due to the retrospective nature of the study.
Acknowledgments
We acknowledge the Society of Obstetrics and Gynecology in Chugoku and Shikoku that supported this study. The physicians and institutions that cooperated in this study are described below:
Yoko Narai, Department of Obstetrics and Gynecology, Shimane Prefectural Central Hospital, Shimane, Japan
Tetsurou Honda, Department of Obstetrics and Gynecology, Kurashiki Central Hospital, Okayama, Japan
Kenji Kai, Department of Obstetrics and Gynecology, National Hospital Organization Fukuyama Medical Center, Hiroshima, Japan
Katsuhiko Tada, Department of Obstetrics and Gynecology, National Hospital Organization Okayama Medical Center, Okayama, Japan
Takashi Harada, Division of Reproductive-Perinatal Medicine and Gynecologic Oncology, Tottori University Faculty of Medicine, Tottori, Japan
Kazuhisa Maeda, Department of Obstetrics and Gynecology, National Hospital Organization Shikoku Medical Center for Children and Adults, Kagawa, Japan
Ryuhei Nagai, Department of Obstetrics and Gynecology, Kochi Health Sciences Center, Kochi, Japan
Koichiro Shimoya, Department of Obstetrics and Gynecology, Kawasaki Medical School, Okayama, Japan
Emiko Abe, Department of Obstetrics and Gynecology, Ehime Prefectural Central Hospital, Ehime, Japan
Yuko Matsubara, Department of Obstetrics and Gynecology, Ehime University Graduate School of Medicine, Ehime, Japan
Susumu Murata, Department of Obstetrics and Gynecology, Yamaguchi University School of Medicine, Yamaguchi, Japan
Hiroshi Miyoshi, Department of Obstetrics and Gynecology, Hiroshima Prefectural Hospital, Hiroshima, Japan
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Iwama N, Ishikuro M, Tanaka K, et al. Epidemiological studies regarding hypertensive disorders of pregnancy: a review. J Obstet Gynaecol Res. 2020; 46(9):1672–7. DOI:10.1111/jog.14383
- Roberts JM. Preeclampsia: what we know and what we do not know. Semin Perinatol. 2000; 24(1):24–28.
- Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. 2019; 134-135:1–10.
- Seki H. Balance of antiangiogenic and angiogenic factors in the context of the etiology of preeclampsia. Acta Obstet Gynecol Scand. 2014; 93(10):959–964.
- Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology. 2004; 145(11):4835–4837.
- Gilbert JS, Gilbert SA, Arany M, et al. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009; 53(2):399–403. DOI:10.1161/HYPERTENSIONAHA.108.123513
- Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt-1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111(5):649–658. DOI:10.1172/JCI17189
- Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008; 21(1):9–23. DOI:10.1080/14767050701830480
- Clayton PE, Cianfarani S, Czernichow P, et al. Management of the child born small for gestational age through to adulthood: a consensus statement of the international societies of pediatric endocrinology and the growth hormone research society. J Clin Endocrinol Metab. 2007; 92(3):804–810. DOI:10.1210/jc.2006-2017
- Saenger P, Czernichow P, Hughes I, et al. Small for gestational age: short stature and beyond. Endocr Rev. 2007; 28(2):219–251. DOI:10.1210/er.2006-0039
- Houk CP, Lee PA. Early diagnosis and treatment referral of children born small for gestational age without catch-up growth are critical for optimal growth outcomes. Int J Pediatr Endocrinol. 2012; 2012(1):11.
- Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995; 38(5):733–739.
- Itabashi K, Mishina J, Tada H, et al. Longitudinal follow-up of height up to five years of age in infants born preterm small for gestational age; comparison to full-term small for gestational age infants. Early Hum Dev. 2007; 83(5):327–333. DOI:10.1016/j.earlhumdev.2006.07.002
- Watanabe K, Matsubara K, Nakamoto O, et al. Outline of the new definition and classification of “hypertensive disorders of pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens Res Pregnancy. 2018; 6(2):33–37. DOI:10.14390/jsshp.HRP2018-014
- Fujita K, Nagasaka M, Iwatani S, et al. Prevalence of small for gestational age (SGA) and short stature in children born SGA who qualify for growth hormone treatment at 3 years of age: population-based study. Pediatr Int. 2016; 58(5):372–376. DOI:10.1111/ped.12859
- Hokken-Koelega AC, De Ridder MA, Lemmen RJ, et al. Children born small for gestational age: do they catch up? Pediatr Res. 1995; 38(2):267–271. DOI:10.1203/00006450-199508000-00022
- Matsumoto M, Nagano N, Awano H, et al. Incidence and neonatal risk factors of short stature and growth hormone treatment in Japanese preterm infants born small for gestational age. Sci Rep. 2019; 9(1):12238. DOI:10.1038/s41598-019-48785-y
- Engström E, Niklasson A, Wikland KA, et al. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005; 57(4):605–610. DOI:10.1203/01.PDR.0000155950.67503.BC
- Hansen-Pupp I, Löfqvist C, Polberger S, et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr Res. 2011; 69(5 Part 1):448–453. DOI:10.1203/PDR.0b013e3182115000
- Cutfield WS, Regan FA, Jackson WE, et al. The endocrine consequences for very low birth weight premature infants. Growth Horm IGF Res. 2004; 14:S130–135.
- Barker DJ, Huppert FA, Baylis N. The developmental origins of well–being. Philos Trans R Soc Lond B Biol Sci. 2004; 359(1449):1359–1366.
- Lawn JE, Blencowe H, Oza S, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014; 384(9938):189–205. DOI:10.1016/S0140-6736(14)60496-7
- Wiedmeier JE, Joss-Moore LA, Lane RH, et al. Early postnatal nutrition and programming of the preterm neonate. Nutr Rev. 2011; 69(2):76–82. DOI:10.1111/j.1753-4887.2010.00370.x
- Zeegers B, Offerhaus P, Peters L, et al. Impact of maternal height on birthweight classification in singleton births at term: a cohort study in the Netherlands. J Matern Fetal Neonatal Med. 2020; 35(16):1–8. DOI:10.1080/14767058.2020.1814246
- Voigt M, Rochow N, Jährig K, et al. Dependence of neonatal small and large for gestational age rates on maternal height and weight – an analysis of the German perinatal survey. J Perinat Med. 2010; 38(4):425–430. DOI:10.1515/jpm.2010.059
- Zhang X, Mumford SL, Cnattingius S, et al. Reduced birthweight in short or primiparous mothers: physiological or pathological? BJOG. 2010; 117(10):1248–1254. DOI:10.1111/j.1471-0528.2010.02642.x
- Roberts G, Cheong JL. Long-term growth and general health for the tiniest or most immature infants. Semin Fetal Neonatal Med. 2014; 19(2):118–124.
- Sohlberg S, Stephansson O, Cnattingius S, et al. Maternal body mass index, height, and risks of preeclampsia. Am J Hypertens. 2012; 25(1):120–125. DOI:10.1038/ajh.2011.175
- Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003; 111(5):986–990.
- Mitsui T, Masuyama H, Eguchi T, et al. Sex differences in early growth during the first three years of life in offspring from mothers with pregnancy-induced hypertension. Pregnancy Hypertens. 2016; 6(4):361–366. DOI:10.1016/j.preghy.2016.08.238
- Vatten LJ, Romundstad PR, Holmen TL, et al. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol. 2003; 101(3):529–533. DOI:10.1097/00006250-200303000-00019
- Ogland B, Vatten LJ, Romundstad PR, et al. Pubertal anthropometry in sons and daughters of women with preeclamptic or normotensive pregnancies. Arch Dis Child. 2009; 94(11):855–859. DOI:10.1136/adc.2008.150870