ABSTRACT
Aim
To compare oral nifedipine and intravenous labetalol in the treatment of acute severe hypertension in pregnancy (SHP).
Methods
The primary outcomes were the required time to achieve target blood pressure (RTATBP), systolic blood pressure (SBP) and diastolic BP (DBP) after treatment, secondary outcomes were the number of doses (NoD) and adverse events (AEs).
Results
There was no difference between oral nifedipine and intravenous labetalol in SBP, DBP, and AE. However, oral nifedipine provided less RTATBP and NoD.
Conclusion
Oral nifedipine was associated with less RTATBP and NoD and otherwise did not differ from intravenous labetalol.
Introduction
Hypertensive disorders of pregnancy (HDP), which include chronic hypertension with or without superimposed pre-eclampsia/eclampsia, gestational hypertension, pre-eclampsia with or without severe features, hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, or eclampsia, contribute to a significant risk of maternal, fetal and newborn morbidity and mortality (Citation1). Specifically, 25000 maternal deaths due to HDP occur yearly in Africa, 22000 in Asia, 3,800 in Latin America and the Caribbean, and 150 in industrialized countries (Citation2). Further, HDP complicates 5% to 10% of pregnancies worldwide (Citation3) and accounts for 18% of maternal deaths (Citation4). Pregnant women with HDP may experience central nervous system dysfunction, hepatocellular injury, thrombocytopenia, acute disseminated intravascular coagulation, oliguria, pulmonary edema, cerebrovascular events, and placental abruption, particularly when they progress to pre-eclampsia (Citation5–7). Therefore, deciding the optimal treatment strategy for this condition is essential.
Severe hypertension during pregnancy (SHP) is defined as systolic blood pressure (SBP)≥ 160 mmHg or diastolic blood pressure (DBP) ≥ 110 mmHg (Citation8–10). SHP is an emergency requiring immediate anti-hypertensive drugs to lower the BP, further reducing the risk of consequences associated with SHP. In the current clinical practice guidelines (CPGs) such as the American College of Obstetricians and Gynecologists (ACOG) bulletin (Citation11), Society of Obstetricians and Gynecologists of Canada (SOGC) guidelines (Citation12), and National Institute for Health and Clinical Excellence (NICE) guidelines (Citation13), medications as intravenous labetalol, hydrazine, and oral nifedipine was recommended as the first-line treatment for acute SHP. However, further research is needed to determine the most effective drug since there is no sufficient evidence regarding the comparative efficacy and safety of these drugs (Citation6,Citation14,Citation15). Notably, the preferred medication for treating SHP has traditionally been hydralazine. However, over the past decade, there has been an increase in maternal and infant problems associated with hydralazine use, and confidence in hydralazine has decreased (Citation16,Citation17). Therefore, it is necessary to determine the difference between oral nifedipine and intravenous labetalol in treating SHP.
Until now, several studies (Citation18–20) have investigated the comparative efficacy and safety of oral nifedipine versus intravenous labetalol in treating acute SHP; however, many studies differed in methodology, such as study design and inclusion and exclusion criteria. According to the study by Magee et al (Citation16), oral nifedipine and intravenous labetalol had similar therapeutic efficacy. In contrast, a study by Chen et al. revealed a significant difference between intravenous labetalol and oral nifedipine in therapeutic efficacy in treating acute SHP (Citation21). Theoretically, intravenous administration will lead to more adverse events, although it may generate more rapid action than oral administration (Citation22). Considering the continued debate regarding the therapeutic efficacy and safety between oral nifedipine and intravenous labetalol in treating SHP, we conducted this meta-analysis to comprehensively investigate the comparative therapeutic efficacy and safety of oral nifedipine with intravenous labetalol for the treatment of SHP.
Methods
We designed this meta-analysis in accordance with the Cochrane Handbook for intervention review (Citation23). We reported all results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Citation24). However, we did not register the formal protocol of this meta-analysis in any public platform. All analyses were based on previously published studies; thus, no ethical approval and patient consent are required because no patient was directly involved in our meta-analysis.
Search strategy
Two independent reviewers electronically searched MEDLINE (via PubMed), Scopus, Web of Science (WoS), China National Knowledge Infrastructure (CNKI), and the Cochrane Central Register of Controlled Trials (CENTRAL) databases using the following terms, including “nifedipine,” “labetalol,” “hypertension,” “pregnancy” and “random.” We systematically searched the individual database on July 2021. Two independent reviewers conducted a preliminary analysis, removed the duplicates, screened the titles and abstracts for relevance, and determined the articles to be excluded. We then reviewed the full texts of included studies. References were manually checked to identify additional potentially eligible studies. The complete search strategy is summarized in Table S1. Any disagreements between the two reviewers were resolved by discussion.
Selection criteria
Studies were included if they:
(a) were randomized-controlled trials (RCTs) published in English or Chinese;
(b) included pregnant patients who were confirmed to have acute SHP according to the criteria proposed by the International Society for the Study of Hypertension in Pregnancy (Citation8);
(c) enrolled patients who were assigned to receive oral nifedipine or intravenous labetalol;
(d) reported at least one of the outcomes of interesting (SBP and DBP after treatment, required times to achieve target BP [RTATBP], the number of doses [NoD], and adverse events [AEs]).
Studies were excluded if they:
(a) were case reports, meta-analyses, letters to the editor;
(b) did not individually prescribe oral nifedipine or intravenous labetalol to patients;
(c) enrolled patients who were confirmed to have antenatal or postpartum SHP.
Data extraction and review
Two reviewers independently extracted essential data. For each study, the following data were collected: date of publication, the first author’s surname, origin, the number of patients enrolled and randomized in each study, mean age (years), mean gestational age (weeks), recruitment period, outcomes of interest, and the detailed information of the risk of bias.
Quality assessment
Cochrane risk of bias assessment tool (Citation25) was applied to assess the methodological quality of all studies. The risk of bias in each study was rated as “high,” “low,” or “unclear” risk according to the match level between actual information and the assessment criteria. The overall methodological level was rated as high if all items were labeled as low risk, moderate if at least one item was labeled as an unclear risk but no items were labeled as high risk, and moderate if at least one item was marked as high risk.
Statistical analysis
Cochrane Q-test was used to evaluate heterogeneity (Citation26) qualitatively, and I2 statistic was used to quantify heterogeneity (Citation26). We used the random-effects model to calculate all pooled estimates because variations across studies could not be eliminated in real-world settings (Citation23). We used the leave-one-out method to conduct a sensitivity analysis for the outcome with substantial statistical heterogeneity. We used the mean difference (MD) with a corresponding 95% confidence interval (CI) to express the estimates of continuous variables, including SBP after treatment, RTATBP, and the NoD. We used the risk ratio (RR) with corresponding 95% CI to express the estimates for dichotomous variables. All statistical analyses were conducted by using Review Manager (RevMan), version 5.0 (Nordic Cochrane Center). Two-tailed P value < 0. 05 indicated statistical significance.
Publication bias examination
The funnel plots were used to detect publication bias by evaluating the asymmetry when the accumulated number of eligible studies for individual outcomes was more than 10 (Citation13,Citation27,Citation28).
Results
Search results
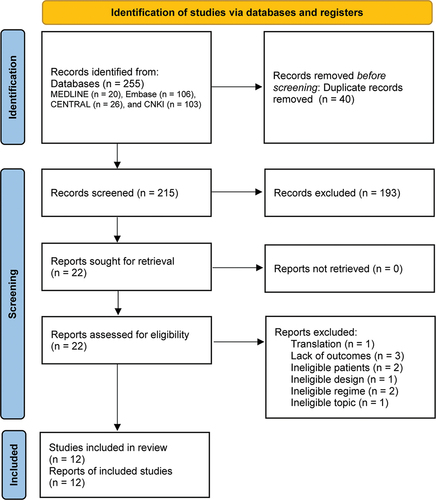
An electronic search yielded 255 records. After a thorough review, 12 eligible studies (Citation19,Citation20,Citation29–38) met our selection criteria. shows the flow diagram reporting the selection process and the search results.
Study characteristics of enrolled studies
The main features of these enrolled studies are summarized in . All the studies were published between 1999 and 2019. Most of the enrolled studies were conducted in India and China. The sample size of included studies ranged from 30 to 221, with a total sample size of 1088. Three studies reported SBP after treatment; nine reported RTATBP, nine reported NoD, and nine reported AEs.
Table 1. Characteristics of the included studies (n = 12).
Quality assessment
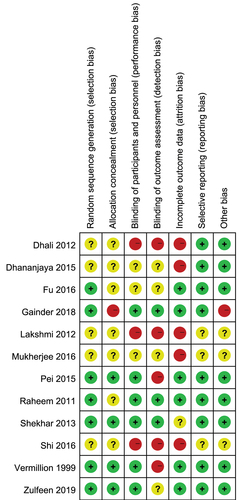
The results of the risk of bias assessment based on the Cochrane risk of bias assessment tool (Citation25) are reported in . Eleven eligible studies were rated as a low or unclear risk in the selection bias domain. Three and five studies were rated as high risk in performance and detection bias domains, respectively. Five studies were negatively affected by attrition bias. Only one study was rated as high risk in other bias sources domain due to insufficient sample size. The overall methodological quality of all enrolled studies was rated as low to moderate.
SBP and DBP after treatment
Three studies with 341 patients (170 patients in the oral nifedipine group and 171 in the intravenous labetalol group) reported the data of SBP and DBP after treatment. The result of the heterogeneity examination was not statistically significant (P = 0.30, I2 = 17%) for SBP but was statistically significant for DBP (P < 0.10, I2 = 86%). Meta-analysis of three studies showed no significant difference between oral nifedipine and intravenous labetalol in reducing SBP (MD, 0.51; 95% CI, −2.56 to 3.58; P = 0.74) and DBP (MD, 1.12; 95% CI, −2.39 to 4.63; P = 0.53), which was illustrated in Figure S1. Meanwhile, for the meta-analysis of DBP, sensitivity analysis did not significantly change the pooled result (Table S2), indicating that the pooled result may not be negatively influenced by statistical heterogeneity.
RTATBP
Nine studies involving 678 patients (339 in the oral nifedipine group and 339 in the intravenous labetalol group) reported the data on the RTATBP. Meta-analysis of nine studies suggested that RTATBP in patients receiving oral nifedipine was significantly less than in patients receiving intravenous labetalol (MD, −10.21; 95% CI, −16.53 to −3.89; P = 0.002), which was illustrated in . However, nine studies had substantial statistical heterogeneity (P < 0.001, I2 = 92%). Sensitivity analysis did not significantly impact heterogeneity based on the leave-one-out strategy (Table S2). Meanwhile, sensitivity analysis did not change the pooled result substantially (Table S2), indicating that statistical heterogeneity may not negatively influence the result.
Number of doses
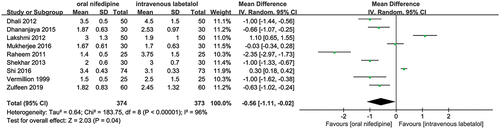
A total of nine studies that enrolled 747 patients (374 in the oral nifedipine group and 373 in the intravenous labetalol group) reported the number of doses. Pooled results revealed that the number of doses in patients receiving oral nifedipine was significantly less than in patients receiving intravenous labetalol (MD, −0.56; 95% CI, −1.11 to −0.02; P = 0.04), which was illustrated in . However, these nine studies had significant statistical heterogeneity regarding this outcome (P < 0.001, I2 = 96%). Sensitivity analysis based on the leave-one-out strategy did not significantly reduce the level of statistical heterogeneity (Table S2) but significantly changed the pooled results after removing some studies (Table S2).
AEs
Nine studies that enrolled 898 patients (449 in each group) reported the incidence of AEs after treatment, and the pooled result revealed no statistical difference between the two groups in terms of AEs (RR, 1.00; 95% CI, 0.66 to 1.52; P = 1.00), which was illustrated in Figure S2. The result of the heterogeneity examination was statistically significant (P = 0.04, I2 = 51%). Sensitivity analysis revealed a significant reduction in heterogeneity after removing some studies (Table S2); however, the pooled result was not significantly changed (Table S2), indicating the result was robust despite substantial statistical heterogeneity.
Publication bias
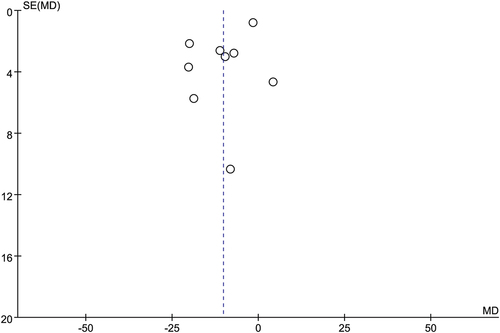
Although the accumulated number of eligible studies for RTATBP, NoD, and AEs was less than 10, we still drew a funnel plot to inspect the possibility of publication bias qualitatively. Symmetric funnel plots indicated the risk of publication bias in terms of RTATBP (Figure S3), NoD (Figure S4), and AEs (Figure S5).
Discussion
Although oral nifedipine and intravenous labetalol have been recommended as the first-line strategy for treating SHP (Citation11), the relative efficacy and safety between the two treatments remain controversial (Citation39). The present meta-analysis was carried out to compare further the efficacy and safety of oral nifedipine and intravenous labetalol for treating SHP. This meta-analysis included 12 studies involving 1088 patients in the final data analysis. Our results revealed that oral nifedipine did not significantly differ from intravenous labetalol in reducing SBP and DBP, and these two therapeutic strategies were comparable regarding the risk of causing AEs. However, oral nifedipine was associated with significantly less RTATBP and NoD than intravenous labetalol.
The treatment of hypertension during pregnancy mainly aims to lower blood pressure, gain enough time to promote fetal maturity, and ultimately prevent dangerous complications in pregnant women, such as hypertensive encephalopathy and heart failure, and protect the health of mothers and children (Citation40–42). Therefore, it is crucial to lowering BP to safe levels (Citation43). The current meta-analysis revealed no significant difference in the efficacy of oral nifedipine and intravenous labetalol in reducing SBP and DBP, consistent with several previous meta-analyses (Citation14,Citation44,Citation45). In 2013, Duley et al. conducted a meta-analysis to compare the efficacy and safety of drugs for treating very high blood pressure during pregnancy and found that oral nifedipine was comparable to intravenous labetalol. Furthermore, the network meta-analysis by Sridharan et al (Citation45). also showed no difference in efficacy in controlling the BP in pregnancy for oral nifedipine compared to intravenous labetalol. In their meta-analysis, Alavifard et al (Citation44). also found that oral nifedipine was not superior to intravenous labetalol for successfully treating severe hypertension. However, one meta-analysis (Citation6) also yielded inconsistent results with our finding, revealing that oral nifedipine was more efficacious than intravenous labetalol in lowering the BP to safer levels. However, these authors also stated that their results must be interpreted cautiously, as sensitivity analysis showed their results lack robustness, and heterogeneity between studies became significant when using risk differences to express pooled results. A recent meta-analysis (Citation46) also revealed that oral nifedipine had the highest therapeutic success rate in controlling hypertension during pregnancy compared to intravenous labetalol. We must acknowledge that the definition of severe hypertension and target blood BP varied widely between the studies because several international guidelines define BP targets in pregnancy differently (Citation39), which will inevitably harm the evaluation of the therapeutic effect. Additionally, it is noted that meta-analysis of DBP yielded significant statistical heterogeneity (86%); one of the three studies had a dominating number of patients contributing to the heterogeneity because this study reported inconsistent results with another two studies (Citation33). Therefore, more studies with larger sample sizes are necessary to evaluate further these two drugs’ comparative efficacy in lowering DBP.
Pregnant women diagnosed with SHP need to be treated with anti-hypertensive medications immediately to lower the very high BP to safe levels timely (Citation43). Therefore, drugs that can quickly reduce very high BP to target BP with fewer doses are more beneficial for treating SHP (Citation47). This meta-analysis found that oral nifedipine achieved target blood pressure faster in less time and with less dose than intravenous labetalol. Some previous meta-analyses have also partially found consistent results with our meta-analysis. In 2015, Holbrook et al. (Citation48) performed a meta-analysis and revealed that oral nifedipine was associated with a shorter time to achieve target BP than intravenous labetalol. Furthermore, the meta-analysis by Shi et al. (Citation18) found that oral nifedipine required a significantly shorter time and fewer doses to achieve the target BP than intravenous labetalol. One reason may be that nifedipine has a rapid onset of action, oral bioavailability, and a long duration of action (Citation49). Notably, meta-analyses of RTATBP (92%) and NoD (96%) all yielded significant statistical heterogeneity, which means the confounders such as race, duration of treatment, and severity of disease could have biased our results (Citation50). Two eligible studies (Citation34,Citation35) recruited patients with hypertensive emergencies, thus inevitably introducing heterogeneity due to variations in clinical characteristics (Citation50). In addition, variations in the doses and origins were also important contributors to the significant statistical heterogeneity (Citation39,Citation50).
In addition to considering therapeutic effects when choosing the appropriate drugs for treating SHP, it is also important to avoid adverse events (Citation51). In the current meta-analysis, we found that the incidence of AEs did not differ significantly between patients who received oral nifedipine and those who received intravenous labetalol. Several previous studies have confirmed our current results. Duley et al. (Citation14) found no difference in the risk of maternal hypotension and AEs between oral nifedipine and intravenous labetalol. In addition, another two meta-analyses by Alavifard et al. (Citation44) and Awaludin et al. (Citation46) have also consistently revealed a comparable risk of AEs between oral nifedipine and intravenous labetalol.
Our study also has some limitations. The first limitation is the small sample size. Second, although previous meta-analyses have pointed out the importance of investigating the effects of different doses on therapeutic efficacy and safety, we did not conduct a subgroup analysis because of the limited number of eligible studies (Citation6). Therefore, we suggested investigating the comparative efficacy and safety of different dose regimens in network meta-analysis when adequate eligible studies were available. Third, most of the studies were rated as at high risk, which may impair estimates’ reliability and robustness. Fourth, we did not register the formal protocol of the current meta-analysis in any public platform; however, we conducted it according to the methodological framework proposed by the Cochrane Collaboration and reported all results following the PRISMA checklist. Finally, this meta-analysis did not evaluate the difference between oral nifedipine and intravenous labetalol in pregnant and fetal outcomes. Therefore, future studies are warranted to evaluate the impact of oral nifedipine and intravenous labetalol on these outcomes.
Conclusions
Based on the currently available evidence, we conclude that intravenous labetalol and oral nifedipine may be comparable in therapeutic efficacy and safety for treating SHP; however, oral nifedipine requires less time than intravenous labetalol to achieve target BP. However, further studies are warranted to validate our findings because the present meta-analysis has limitations and investigates the effects of different doses on therapeutic efficacy and safety.
Author contributions
Minghui Ou and Yan Yu conceived and supervised the study; Yan Yu and Futao Zhang designed experiments; Shichao Cui and Shibo Zhao performed experiments; Minghui Ou and Futao Zhang analyzed data; Minghui Ou Yu and Futao Zhang wrote the manuscript; Yan Yu made the manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Contribution to the field statement
These findings showed that nifedipine and labetalol have similar clinical effects, while nifedipine is more efficient than labetalol for treating pregnancy-induced hypertension.
Supplemental Material
Download Zip (1.1 MB)Disclosure statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
No datasets were generated for this study
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2209637.
Additional information
Funding
References
- Luger RK, Kight BP. Hypertension in Pregnancy. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–10.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. DOI:10.1016/S0140-6736(12)61728-0
- Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074.
- Duro-Gomez J, Rodriguez-Marin AB, Gimenez de Azcarete M, et al. A trial of oral nifedipine and oral labetalol in preeclampsia hypertensive emergency treatment. J Obstet Gynaecol. 2017;37(7):864–866. DOI:10.1080/01443615.2017.1308321
- Shekhar S, Gupta N, Kirubakaran R, et al. Oral nifedipine versus intravenous labetalol for severe hypertension during pregnancy: a systematic review and meta-analysis. BJOG: An Int J Obstetrics & Gynaecol. 2016;123(1):40–47.
- Nelson-Piercy C LWM. Nelsonpiercy C ea. 9 pregnancy and chronic hypertension: nifedipine vs labetalol as antihypertensive treatment (the panda study): anti-hypertensive medications. Pregnancy Hypertens Int J Womens Cardiovasc Health. 2016;6(3):141–.
- Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: iSSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
- Committee A. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–25.
- Lowe SA, Brown MA, Dekker GA, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust NZ J Obstetrics Gynaecol. 2009;49(3):242–246. DOI:10.1111/j.1479-828X.2009.01003.x
- Bertani H, Gelmini R, Del Buono MG, et al. Literature overview on artificial liver support in fulminant hepatic failure: a methodological approach. Int J Artif Organs. 2002;25(10):903–910. DOI:10.1177/039139880202501002
- Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4(2):105–145.
- National Collaborating Centre for Ws. Children’s H. National Institute for Health and Clinical Excellence: guidance. Hypertension in Pregnancy: the Management of Hypertensive Disorders During Pregnancy. London: RCOG Press Copyright © 2022, StatPearls Publishing LLC; 2022.
- Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2013;2013(7). DOI:10.1002/14651858.CD001449.pub3
- Firoz T, Magee LA, MacDonell K, et al. Oral antihypertensive therapy for severe hypertension in pregnancy and postpartum: a systematic review. BJOG. 2014;121(10):1210–1218. doi: 10.1111/1471-0528.12737. discussion 20.
- Magee LA, Cham C, Waterman EJ, et al. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327(7421):955–960.
- Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318(7194):1332–1336.
- Shi Q, Leng W, Yao Q, et al. Oral nifedipine versus intravenous labetalol for the treatment of severe hypertension in pregnancy. Int J Cardiol. 2015;178:162–164.
- Zulfeen M, Tatapudi R, Sowjanya R. IV labetalol and oral nifedipine in acute control of severe hypertension in pregnancy-A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2019;236:46–52.
- Dhananjaya BS, Jamuna R. Oral nifedipine versus intravenous labetalol in hypertensive emergencies of pregnancy: a randomised trial. Res J Pharm Biol Chem Sci. 2015;6:1673–1681.
- Chen Q, Xiao XR, Zhao MZ, et al. The antihypertensives, nifedipine and labetalol prevent endothelial cell activation in response to placental extracellular vesicles released from 1st trimester placental explants that had been treated with preeclamptic sera. Pregnancy Hypertension: An Int J Women Cardiovascular Health. 2016;6(3):140. DOI:10.1016/j.preghy.2016.08.008
- Yang CZ, Zhou Y, Ke M, et al. Effects of postoperative adjuvant steroid therapy on the outcomes of biliary atresia: a systematic review and updated meta-analysis. Front Pharmacol. 2022;13:956093.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane; 2021 [updated 2021 Feb; Accessed 2023 Apr 10]. www.training.cochrane.org/handbook
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. DOI:10.1136/bmj.d5928
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- Page MJ, McKenzie JE, Higgins JPT. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open. 2018;8(3):e019703.
- Palma Perez S, Delgado Rodriguez M. Practical considerations on detection of publication bias. Gac Sanit. 2006;20(Suppl 3):10–16.
- Dhali B, Bhattacharya S, Ganguly R, et al. A randomized trial of intravenous labetalol and oral nifedipine in severe pregnancy induced hypertension. Int J Reprod Contraception Obstetrics Gynecology. 2012;1:42–46.
- Fu ZM, Wang AL, Pei XY. Comparative effects of magnesium sulfate plus nifedipine or labetalol for the treatment of pregnancy-induced hypertension [in Chinese]. Hainan Med J. 2016;27(14):2346–2348.
- Gainder S, Thakur M, Saha SC, et al. To study the changes in fetal hemodynamics with intravenous labetalol or nifedipine in acute severe hypertension. Pregnancy Hypertens. 2019;15:12–15.
- Mukherjee S, Khan S, Jain U. A comparative evaluation of intravenous labetalol versus oral nifedipine for control of severe pregnancy-induced hypertension with low-dose regimen. Int JMed Sci Public Health. 2015;5(6):1.
- Pei YX, Zhang XY, Li JC, et al. Clinical effect of nifedipine and labetalol in the treatment of severe pregnancy-induced hypertension [in Chinese]. Chinese J Clinical Rational Drug Use. 2015;8(22):91–92.
- Raheem IA, Saaid R, Omar SZ, et al. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomised trial. BJOG. 2012;119(1):78–85.
- Sathya Lakshmi B, Dasari P. Oral nifedipine versus intravenous labetalol in hypertensive urgencies and emergencies of pregnancy: a randomized clinical trial. Obstet Med. 2012;5(4):171–175.
- Shekhar S, Sharma C, Thakur S, et al. Oral nifedipine or intravenous labetalol for hypertensive emergency in pregnancy: a randomized controlled trial. Obstet Gynecol. 2013;122(5):1057–1063.
- Shi DD, Yang FZ, Zhou L, et al. Oral nifedipine vs. intravenous labetalol for treatment of pregnancy-induced severe pre-eclampsia. J Clin Pharm Ther. 2016;41(6):657–661.
- Vermillion ST, Scardo JA, Newman RB, et al. A randomized, double-blind trial of oral nifedipine and intravenous labetalol in hypertensive emergencies of pregnancy. Am J Obstet Gynecol. 1999;181(4):858–861.
- Wu HZ, Cheng Y, Yu D, et al. Different dosage regimens of nifedipine, labetalol, and hydralazine for the treatment of severe hypertension during pregnancy: a network meta-analysis of randomized controlled trials. Hypertens Pregnancy. 2022;41(2):126–138.
- Hussein M, Mooij JM, Roujouleh H. Factor analysis, including antihypertensive medication, of the outcome of pregnancy in pregnancy-associated hypertension. Kidney & Blood Pressure Res. 2001;24(2):124–128.
- Mabie WC, Gonzalez AR, Sibai BM, et al. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987;70(3 Pt 1):328–333.
- Mustafa R, Ahmed S, Gupta A, et al. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:105918.
- Practice CoO. Committee opinion no. 692: emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstetrics Gynecol. 2017;129(4):e90–5. DOI:10.1097/AOG.0000000000002019.
- Alavifard S, Chase R, Janoudi G, et al. First-line antihypertensive treatment for severe hypertension in pregnancy: a systematic review and network meta-analysis. Pregnancy Hypertens. 2019;18:179–187.
- Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(9):1906–1916.
- Awaludin A, Rahayu C, Daud NAA, et al. Antihypertensive medications for severe hypertension in pregnancy: a systematic review and meta-analysis. Healthcare (Basel). 2022;10(2):325.
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy: the task force for the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(34):3165–3241. DOI:10.1093/eurheartj/ehy340
- Holbrook B, Nirgudkar P, Mozurkewich E. 574: efficacy of hydralazine, labetalol, and nifedipine for the acute reduction of severe hypertension in pregnancy: a systematic review. Am J Obstet Gynecol. 2015;212(1, Supplement):S286–7.
- Webster LM, Myers JE, Nelson-Piercy C, et al. Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension (Dallas, Tex: 1979). 2017;70(5):915–922. DOI:10.1161/HYPERTENSIONAHA.117.09972
- Alavifard S, Chase R, Chaumont A, et al. First-line antihypertensive treatment for severe hypertension in pregnancy: a systematic review and network meta-analysis. Pregnancy Hypertens. 2019;18:179–187.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. 2011.