ABSTRACT
Objective
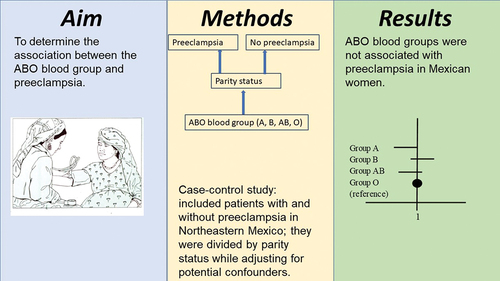
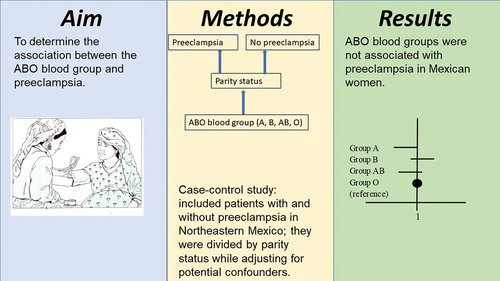
To determine the association between the ABO blood group and preeclampsia.
Methods
This is a case-control study that included patients with (n = 253) and without (n = 457) preeclampsia/eclampsia in Northeastern Mexico. Data were obtained from electronic medical records. Binary multiple logistic regression analysis was used for analyzing the association between the ABO blood group and preeclampsia according to parity status while adjusting for potential confounders.
Results
Blood groups A, B, and AB showed adjusted odds ratios of 0.6 (95%CI 0.3-1.0), 1.1 (95%CI 0.6-2.2), and 0.3 (95%CI 0.1-1.1) in multiparous women, respectively. No association was found in nulliparous women either.
Conclusions
ABO blood groups were not associated with preeclampsia in Mexican women.
Introduction
Preeclampsia is diagnosed by the presence of de novo hypertension after the 20th week of gestation accompanied by proteinuria and/or evidence of maternal acute kidney injury, liver dysfunction, neurological features, hemolysis, thrombocytopenia, or fetal growth restriction (Citation1). It is a public health problem that complicates 5%-7% of pregnancies worldwide (Citation2) and causes 16% and 25% of maternal deaths in the United States (Citation3) and Latin America (Citation4), respectively. Preeclampsia is associated with an increased rate of very low neonatal birth weight, as high as 50%, and the risk of neonatal death is up to 11 times greater than that of patients without preeclampsia (Citation5). Several risk factors for preeclampsia have been described, some of which are present before the index pregnancy (chronic hypertension, diabetes, obesity, and previous preeclampsia), while others are linked to it (multiple gestation, assisted reproduction therapies, antiphospholipid syndrome, and male fetal sex) (Citation1,Citation2–9). In recent years, blood A and B antigens (blood groups A and B) have been implicated in the pathophysiology of preeclampsia. The various pathological mechanisms include the following: a) an increase in von Willebrand factor, whose prothrombotic activity triggers or exacerbates pathophysiological events related to the disease (Citation10), b) a reduction in the placental protein PP13 (Citation11–13), and c) an increase in E-selectin, tumor necrosis factor-alpha, and intercellular adhesion molecule-1 bioavailability (Citation12). The pathological mechanism of preeclampsia in blood group O is unclear. Despite this biological linkage, relevant studies have shown contradictory results. Some studies have shown an increased risk in patients of blood groups AB (Citation11,Citation12-14–Citation18), A (Citation14), B (Citation19), and O (Citation20,Citation21), while others did not find any association (Citation22–24). A consistent association would make it possible to easily categorize those women at risk since the determination of ABO groups is part of the routine clinical practice of primary pregnancy care.
This study aimed to determine the association between ABO blood groups and preeclampsia in a Mexican population. This information may contribute to the identification of patients at a high risk of preeclampsia during the planning stage of pregnancy or at the first antenatal visit to allow for the implementation of preventive and treatment interventions against preeclampsia.
Methods
Design, patients, and procedures
This case-control study was conducted between 2017 and 2020 in mestizo patients from northeastern Mexico. Cases comprised patients diagnosed with preeclampsia/eclampsia by certified gynecologists who followed national and international guidelines: hypertension after the 20th week of gestation and proteinuria. In the absence of the latter, they considered signs of systemic severity such as thrombocytopenia and liver, kidney, or neurological dysfunction (n = 253) (Citation25,Citation26). The control group included patients who did not meet the diagnostic criteria for preeclampsia (n = 457). Cases and controls who visited our institution for the first antenatal visit before the 20th week of pregnancy were consecutively enrolled. Six eligible cases and 49 eligible controls were excluded because information on blood group type and other variables was unavailable. An initial sample size of 186 cases and 372 controls was calculated based on an odds ratio of 2.42 (for group AB) (Citation15), a percentage of exposure in cases of 12.9%, and statistical power of 80%. The final sample was composed of 253 cases and 457 controls, increasing the statistical power to 87.3%. The protocol adhered to the institutional, national, and international standards and regulations on research ethics and was authorized by the Local Ethics and Research Committee No. 1912 of the Mexican Institute of Social Security (approval number R-2018-1912-021). All data were recorded anonymously, respecting the confidentiality of the patients. Since the information necessary for the study was collected only from electronic medical records, informed consent was not required from patients.
Study variables
The following clinical data were collected: systolic and diastolic blood pressure at the first antenatal visit (first trimester), history of gestational diabetes, and gestational weight gain (weight difference between the last and first antenatal visit). Obstetrical data included the number of pregnancies, inter-pregnancy interval in multiparous women, current multiple pregnancy, number of antenatal visits, fetal sex, and gestational age at the last antenatal visit. Medical history data included a history of preeclampsia/eclampsia, hypertension, type 2 diabetes, pre-pregnancy overweight/obesity (body mass index≥25 kg/m2), and aspirin use during pregnancy. Sociodemographic data included information on maternal age, education, and occupation. Neonatal data included the gestational age, birth weight, and one- and five-minute Apgar scores. Laboratory data included the blood group type and Rh factor. Blood groups were determined using the hemagglutination technique. All data were collected from electronic medical records. The mean arterial pressure was calculated from the systolic and diastolic blood pressures using the following formula: ([systolic blood pressure - diastolic blood pressure/3] + diastolic blood pressure).
Data analysis
Measures of central tendency and dispersion were estimated for quantitative variables and proportions for categorical variables. Cases and controls were compared using the Mann-Whitney U test, Kruskal-Wallis test, or chi-square test, depending on the scale of the variable. Univariate and multivariate odds ratios (OR) with 95% confidence intervals (CIs) were determined using binary multiple logistic regression. Two models were run separately, one for nulliparous patients and the other for multiparous patients. The blood group was used as an independent variable after classifying patients according to blood type (A, B, AB, and O; type O was used as the reference group). Preeclampsia/eclampsia was the dependent variable (1 = yes), and confounding variables varied according to nulliparity status (the model for multiparous patients included a medical history of preeclampsia (Citation27) and inter-pregnancy interval (Citation28). Statistical significance was set at p < 0.05.
Results
Cases and controls had similar maternal (27.8 ± 5.8 vs 28.2 ± 5.7 years, p = 0.23) and gestational ages at the first antenatal visit (13.2 ± 5.6 vs 13.6 ± 7.5 weeks, p = 0.30), and an equivalent number of antenatal visits (8.2 ± 3.5 vs 8.5 ± 4.3, p = 0.85). Preeclampsia was of early onset (before the 34th week of gestation) in thirteen (5.1%) cases. The proportion of patients with an educational level of junior high or less, and medical history of preeclampsia/eclampsia, hypertension, pre-pregnancy overweight/obesity, and male fetal sex was higher among cases, while the economically active occupation was more common among controls (). Compared to controls, cases had higher systolic blood pressure, diastolic blood pressure, and mean arterial pressure at the first antenatal visit, more weight gain, and a higher proportion of patients with a history of gestational diabetes; they had lower gestational age at birth, birth weight, and Apgar at 1 and 5 minutes (). The mean birth weight in the early-onset preeclampsia group was 1563.01 g ±575.05 g, in the late preeclampsia group was 2755.3 g ±477.8 g, and in controls was 3218.2 grams ±364.3 g (p < 0.001).
Table 1. Comparison of sociodemographic, medical, and obstetric characteristics between pregnant women with and without preeclampsia/eclampsia.
Table 2. Comparison of blood group and factor type between pregnant women with and without preeclampsia/eclampsia (cases, n = 253; controls, n = 457).
ABO blood groups and preeclampsia
Blood group O was the most frequent type (60.6%), followed by A (22%), B (12.4%), and AB (5.1%). In addition, a positive Rh factor was the most common (97.7%). There was no difference in the frequency of ABO blood groups and Rh factor between cases and controls in multiparous (p = 0.152) or nulliparous women (p = 0.223; ). The unadjusted odds ratios in multiparous women were as follows: A group = 0.7 (95% CI 0.4-1.1); B group = 1.1 (95% CI 0.6-1.9); and AB group = 0.4 (95% CI 0.1-1.1). In nulliparous women, they were as follows: A group = 1.5 (95% CI 0.8-2.8); B group = 1.5 (95% CI 0.5-2.5); and AB group = 2.0 (95% CI 0.7-5.9). The lack of association continued even after adjusting for confounding variables (). Additionally, we identified previous preeclampsia, chronic hypertension, obesity, higher mean blood pressure at the first prenatal visit, and male fetal sex increased the risk of preeclampsia in multiparous women. The last two also increased the risk of preeclampsia in nulliparous women (Table S1 and S2, respectively).
Table 3. Comparison of blood group and factor type between pregnant women with and without preeclampsia/eclampsia (cases, n = 253; controls, n = 457).
Table 4. Crude and adjusted odds ratios between ABO blood group and preeclampsia. Multivariate logistic regression model among multiparous pregnant women (cases, n = 168; controls, n = 288).
Table 5. Crude and adjusted odds ratios between ABO blood group and preeclampsia. Multivariate logistic regression model among nulliparous pregnant women (cases, n = 85; controls, n = 169).
Discussion
This study examined the association between ABO blood groups and preeclampsia in Mexican women, adjusting for multiple confounding factors. Before discussing the main results, it is pertinent to mention that women with preeclampsia had clinical and obstetric characteristics that were consistent with prior knowledge (Citation27,Citation29), that is, they were more exposed than controls to previous preeclampsia, chronic hypertension, higher mean arterial pressure at the first prenatal visit, and preexisting overweight/obesity. In addition, the distribution of the ABO blood groups of the study sample was similar to that reported in the Mexican population (Citation30).
Regarding our research question, we found no association between ABO blood groups and preeclampsia in a Mexican population, which was similar to other studies (Citation22–24), but opposite to others (Citation11,Citation12-14–Citation17-19–Citation21-31). The discrepancy may be due to differences in controlling confounding factors that could favor or vanish the association. Only one of three studies with results of no association (Citation22–24) controlled for maternal age and parity (Citation23). In studies reporting an association, there is also a lot of heterogeneity in terms of adjustment for confounders. Mital et al. (Citation12), Manjunatha et al. (Citation16), and Avci et al. (Citation17) did not adjust for confounding factors. Others did for age (Citation11,Citation14,Citation18,Citation20,Citation21) body mass index (Citation11,Citation14,Citation18,Citation20,Citation21), parity (Citation14,Citation18,Citation20), and residence (Citation11,Citation20). Hiltunen et al. (Citation18) included Factor V Leiden (a genetic disorder characterized by a poor anticoagulant response to activated Protein C and an increased risk for venous thromboembolism) (Citation32). Parity is an important confounding variable because nulliparity is a well-known risk factor for preeclampsia (Citation2,Citation4,Citation26,Citation33,Citation34). Additionally, medical history of preeclampsia (Citation2,Citation4,Citation27) and inter-pregnancy interval (Citation28) only concerns women with two or more pregnancies. So, the association analysis must consider parity status. We did not find an association in nulliparous or multiparous women. Aghasadeghi et al. (Citation23) also found no association after controlling for parity. But Phaloprakarn et al. (Citation14) did; they identified an association between the AB group and preeclampsia (adjusted OR 1.7, 95% CI 1.1, 2.6) and, Elmugabil et al. (Citation20), between the O group and preeclampsia (adjusted OR 1.8, 95% CI 1.1-2.9). Another possible confounder is fetal sex, recently described as a risk factor (Citation8,Citation9). For this reason, we adjusted the association analysis for this variable. Unfortunately, we were unable to identify another study that did the same and could not compare the effect of this variable on the relationship between blood groups and preeclampsia.
This study is important from the point of view of clinical practice, since the inclusion of different risk factors, and not only blood groups, can more accurately represent pregnant women who are seen more frequently in primary care. We considered the analysis of multiple risk factors for preeclampsia available at a routine antenatal visit because they are clinically relevant. And stratification by parity was necessary to differentiate risk factors in women who have already had a pregnancy compared to those who are in their first pregnancy. Clinicians and decision-makers need information on the prevention and early detection of preeclampsia. The present study adds to those that have not shown an association between blood groups and preeclampsia. However, two cohort studies with large sample sizes and good control of potential confounders have shown the opposite (Citation11,Citation14). Undoubtedly, research is still needed for ending the controversy. Also, research focused on clarifying the underlying pathophysiological mechanisms would be of special importance.
Limitations of the study
The number of patients from groups A and AB was limited and could have affected the lack of association. However, the frequency distribution of blood types was consistent with that present in the general population (Citation30) and that reported by other authors (Citation16–19–Citation21–23). Multicenter studies would allow for larger sample sizes of people with less common blood groups. We would have liked to control for the von-Willebrand factor, but this data was not available. Ultrasonographic evaluation of fetal growth restriction was also not available, but the birth weight was significantly lower in the cases.
Conclusions
ABO blood groups were not associated with an increased risk of preeclampsia in Mexican patients after controlling for parity status and multiple well-known risk factors. Studies such as this one contribute to clarifying the role of blood groups and other variables that will allow, in the future, timely identification of patients at risk of preeclampsia. A graphical abstract of the main results has been included to facilitate their understanding ().
Author contributions
HFCF and AMSM participated in research conception and design, data analysis and interpretation, manuscript writing, and critical revision. LAGH, SDGR, JDTB, and FJGG participated in data acquisition and interpretation and critical revision of the manuscript. All authors have seen and approved the final version.
Supplemental Material
Download (19.2 KB)Acknowledgments
We would like to thank the personnel of the Clinical File Department of the General Hospital of Zone No. 6 of the Mexican Institute of Social Security.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2209640
Additional information
Funding
References
- Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy. Hypertens. 2018;72(1):24–7.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia. Circ Res. 2019;124(7):1094–1112.
- Shih T, Peneva D, Xu X, et al. The rising burden of preeclampsia in the United States impacts both maternal and child health. Am J Perinatol. 2015;33(04):329–338.
- World Health Organization. Recomendaciones de la OMS para la prevención y el tratamiento de la preeclampsia y la eclampsia [Internet]. Geneva: World Health Organization; 2014 [(cited 2021 Aug 2). Available from]. https://apps.who.int/iris/bitstream/handle/10665/138405/9789243548333_spa.pdf;jsessionid=D37A2117D549928B69163B08CA7596A2?sequence=1
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6): 544.e1-544.e12. Available from https://linkinghub.elsevier.com/retrieve/pii/S0002937813008594
- Centro Nacional de Excelencia Tecnológica en Salud, Chávez-Nieto E, Díaz-Velazquez MF, Bautista-Herrera BA, et al, editors. Detección, diagnóstico y tratamiento de Enfermedades Hipertensivas del Embarazo. México: Instituto Mexicano del Seguro Social; 2017. Available from. http://www.imss.gob.mx/sites/all/statics/guiasclinicas/058GER.pdf
- Meazaw MW, Chojenta C, Muluneh MD, et al. Systematic and meta-analysis of factors associated with preeclampsia and eclampsia in sub-Saharan Africa. PLoS one. 2020;15:e0237600.
- Jaskolka D, Retnakaran R, Zinman B, et al. Fetal sex and maternal risk of pre-eclampsia/eclampsia: a systematic review and meta-analysis. BJOG an Int J Obstet Gynaecol. 2017;124(4):553–560.
- Mirzakhani H, Weiss ST. Fetal sex and risk of preeclampsia: dose maternal race matter? J Matern Neonatal Med. 2022;35(17):1–9.
- Zhou S. Is ABO blood group truly a risk factor for thrombosis and adverse outcomes? World J Cardiol. 2014;6(9):985. Available from: http://www.wjgnet.com/1949-8462/full/v6/i9/985.htm
- Lee BK, Zhang Z, Wikman A, et al. ABO and RhD blood groups and gestational hypertensive disorders: a population-based cohort study. BJOG an Int J Obstet Gynaecol. 2012;119(10):1232–1237.
- Mital P, Gupta D, Benwal DK, et al. To find any association of maternal blood group as a risk factor for preeclampsia. Int J Community Med Public Health. 2016;3:3445–3449.
- Than NG, Romero R, Meiri H, et al. PP13, maternal ABO blood groups and the risk assessment of pregnancy complications. PLoS ONE. 2011;6(7):6.
- Phaloprakarn C, Tangjitgamol S. Maternal ABO blood group and adverse pregnancy outcomes. J Perinatol. 2013;33(2):107–111.
- Alpoim PN, De Barros Pinheiro M, Junqueira DR, et al. Preeclampsia and ABO blood groups: a systematic review and meta-analysis. Mol Biol Rep. 2013;40(3):2253–2261.
- Manjunatha S, Anita K. The relationship between maternal blood group and preeclampsia. Int J Reprod Contracept, Obstet Gynecol. 2015;4:1749–1752.
- Avci D, Karagoz H, Ozer O, et al. Are the blood groups of women with preeclampsia a risk factor for the development of hypertension postpartum? Ther Clin Risk Manag. 2016;12:617–622.
- Hiltunen LM, Laivuori H, Rautanen A, et al. Blood group AB and factor V leiden as risk factors for pre-eclampsia: a population-based nested case-control study. Thromb Res. 2009;124(2):167–173.
- Burgess A, Johnson TS, Simanek A, et al. Maternal ABO blood type and factors associated with preeclampsia subtype. Biol Res Nurs. 2019;21(3):264–271.
- Elmugabil A, Rayis DA, Ahmed MA, et al. O blood group as risk factor for preeclampsia among Sudanese women. Maced J Med Sci. 2016;4(4):603–606.
- Mahasub N, Boriboonhirunsarn D. Relationship between ABO blood groups and preeclampsia. Hypertens Pregnancy. 2020;39(3):348–353.
- Hentschke MR, Caruso FB, Paula LG, et al. Is there any relationship between ABO/Rh blood group and patients with pre-eclampsia? Pregnancy Hypertens. 2014;4(2):170–173.
- Aghasadeghi F, Saadat M. Association between ABO and Rh blood groups and risk of preeclampsia: a case-control study from Iran. Open Access Maced J Med Sci. 2017;5(2):173–176.
- Okoye HC, Efobi CC, Ugwu AO, et al. ABO blood group as a biomarker of preeclampsia among antenatal clinic attendees in Nigeria. Niger J Clin Pract. 2020;23(5):729–733.
- Instituto Mexicano del Seguro Social. Prevención, diagnóstico y tratamiento de la preeclampsia en segundo y tercer nivel de atención. Guía Práctica Clínica. 2017;1–94. Available from: http://www.cenetec-difusion.com/CMGPC/S-020-08/ER.pdf
- C. A. ACOG guidelines: hypertension in pregnancy. American college of obstetricians. In: Gynecologists, editor. The American college of obstetricians and gynecologists task force of hypertension in pregnancy. (WA): Library of Congress Cataloging-in-Publication Data; 2013. pp. 17–19.
- Cordero-Franco HF, Salinas-Martinez AM, Garcia-Alvarez TA, et al. Discriminatory accuracy of preeclampsia risk factors in a primary care. Arch Med Res. 2018;49:240–247.
- Cormick G, Betrán AP, Ciapponi A, et al. Inter-pregnancy interval and risk of recurrent pre-eclampsia: systematic review and meta-analysis. Reprod Health [Internet]. 2016;13:83. Available from: http://reproductive-health-journal.biomedcentral.com/articles/10.1186/s12978-016-0197-x
- Cordero-Franco HF, Salinas-Martinez AM, Garcia-Alvarez TA, et al. Comparison of the discriminatory accuracy of four risk criteria for preeclampsia. Pregnancy Hypertens. 2018;13:161–165.
- Canizalez-Román A, Campos-Romero A, Castro-Sánchez JA, et al. Blood groups distribution and gene diversity of the ABO and Rh (D) loci in the Mexican population. BioMed Res Int. 2018;2018:1–11.
- Li T, Wang Y, Wu L, et al. The association between ABO blood group and preeclampsia: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:1–10.
- Li Y, Ruan Y. Association of hypertensive disorders of pregnancy risk and factor V leiden mutation: a meta‐analysis. J Obstet Gynaecol Res. 2019;45:1303–1310.
- Nerenberg KA, Johnson JA, Leung B, et al. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of alberta women. J Obstet Gynaecol Canada [Internet]. 2013;35:986–994. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1701216315307866
- Centro Nacional de Excelencia Tecnológica en Salud. Atención integral de la preeclampsia en el segundo y tercer niveles de atención: Secretaría de Salud; 2008. Available from: http://www.cenetec.salud.gob.mx/descargas/gpc/CatalogoMaestro/020_GPC_Preeclampsia/SS_020_08_EyR.pdf