ABSTRACT
Hypertensive pregnancy disorders affect up to 10% of all pregnancies and are associated with an increased future risk of heart disease, chronic hypertension, kidney dysfunction, diabetes, and thromboembolism. Although mechanisms are not yet well understood, endothelial dysfunction, pro-inflammatory and procoagulant states seem to persist in women with a history of preeclampsia many years after a pregnancy complicated by HDP. Moreover, the number and severity of these complications differs according to the type of disorder developed during pregnancy. Lifestyle modifications and long-term follow-up are essential to reduce the risk of developing a disease later in life.
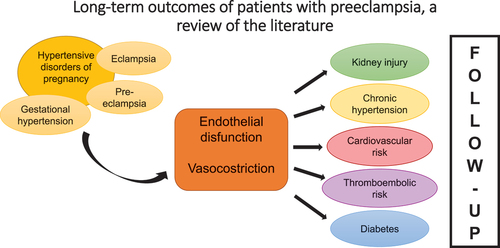
GRAPHICAL ABSTRACT
Introduction
Hypertensive disorders of pregnancy (HDP) include pregnancy-induced hypertension, preeclampsia (PE), eclampsia, and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and affect approximately 10% of all pregnancies (Citation1,Citation2). Pre-eclampsia is a multisystem disorder, defined as the new onset of hypertension generally after 20 weeks’ gestation with evidence of proteinuria and/or acute kidney injury, impaired liver function, severe persistent right upper quadrant or epigastric pain and not accounted for by alternative diagnoses, new-onset cerebral or visual disturbances, thrombocytopenia, or pulmonary edema. Eclampsia is a severe form of pre-eclampsia with maternal seizures in the absence of other causative conditions. Gestational hypertension is defined as de novo hypertension after 20 weeks’ gestation without any of the additional features mentioned for pre-eclampsia (Citation3). Pre-eclampsia, alone, encompasses approximately 2% to 8% of pregnancy-related complications (Citation4). The pathogenesis of pre-eclampsia is still not fully elucidated, and a central hypothesis is that PE results from defective remodeling of placental spiral arteries that leads to placental hypoperfusion. Hypoxia can generate an oxidative stress that triggers an excessive systemic inflammatory response which causes a massive endothelial dysfunction and vasoconstriction. These alterations can lead to systemic hypertension and organ hypoperfusion. Literature is growing, especially underling that these effects on organs persist after pregnancy(Citation5,Citation6). However, what is still unclear is the role of preeclampsia: is PE an independent risk factor for future cardiovascular disease (CVD) or it can be considered an early marker for future cardiovascular disease in women with high-risk profiles (Citation6)? In fact, obesity, metabolic abnormalities, dyslipidemia, insulin resistance, heightened inflammatory responses, hypercoagulable states, and endothelial dysfunction are well-known factors that predispose women to preeclampsia, but also risk factors for cardiovascular diseases(Citation6). Another hypothesis is that the body may not fully recover from the damage to the vascular, endothelial, and metabolic systems associated with preeclampsia and may manifest it with future cardiovascular (CV) events later in life (Citation6). In preeclamptic patients, lipid deposition in the spiral artery walls, which mimic the early stages of atherosclerosis, is more common than in healthy pregnancies (Citation6). Over the years, women with pregnancies complicated by HDP have been found to be at higher risk of developing short-term and long-term CVD after delivery (Citation1,Citation2).
Arnott et al., report that hypertensive disorders of pregnancy are associated with a marked increase in the risk of a maternal cardiovascular event. Patients with early onset of the disease, in particular before the 34th week of gestation, have a greater risk of CV disorders (4.9 times more than healthy women) compared with women with late onset (2.5 times more than healthy women) (Citation7). Moreover, smoking, concomitant gestational diabetes mellitus, or fetal growth restriction (FGR) are conditions leading to a greater risk (Citation7). Pregnancy represents a physiological vascular stress-test and it can be an opportunity for early diagnosis of cardiovascular disorders (Citation8). According to a prospective cohort study conducted in 2022, pregnancies complicated by hypertensive disorders may accurately predict the development of chronic hypertension 6–12 months after delivery.
After pregnancy, the frequency of CV risk factors such as chronic hypertension, kidney dysfunction, dyslipidemia, diabetes, and subclinical atherosclerosis is higher in women who developed HDP. The prevalence and onset of CV risk factors depend on the severity of HDPs and the coexistence of other complications (Citation2).
In this review of the literature, the main risks related to the development of HDP in pregnancy and the possible long-term outcomes will be investigated. In addition, the importance of proper follow-up for these women will be highlighted, even after childbirth.
Kidney injury
The impact of HDP on renal function in the years following pregnancy is widely reported in the literature (Citation7). The association between preeclampsia and renal damage is well documented by several studies (Citation9–12) and may reflect the presence of common risk factors. Potential renal consequences of pre-eclampsia include microalbuminuria (MA), acute kidney disease (AKD), and increased susceptibility to chronic kidney disease (CKD) (Citation2,Citation9,Citation13–16).
MA is a persistent and increased urinary albumin excretion and is a well-known marker of renal dysfunction and a risk factor for CVD (Citation2). A meta-analysis by McDonald et al. of 2010 reported that women with a history of preeclampsia had an increased risk of microalbuminuria. In particular, these patients had a 4-fold increased risk of developing it 7.1 (95% CI; 4.5–9.7) years after delivery compared to women with uncomplicated pregnancies (Citation13). A recent Canadian follow-up study examined the risk of end-stage renal disease in 1.5 million women (median follow-up 16.2 years; IQR 13.3–18.3). This study reported an increased risk in women with preeclampsia or eclampsia during pregnancy. The risk also appeared to be higher when associated with other risk factors such as FGR fetus, severe obesity, and CVD (Citation17). Other studies are in line with these results (Citation14–16). Although most reports agree on the increased risk of end-stage renal disease in women with previous preeclampsia, there is no unanimity in the literature about the origin of the risk of acute renal disease. Indeed, while AKD is a potential complication of preeclampsia, it is not known whether preeclampsia causes AKD in the long term (Citation9).
Considering the above, most studies agree on the need for follow-up for kidney disease in the immediate post-pregnancy years in women with prior pre-eclampsia. Early diagnosis of chronic kidney disorders would allow for early intervention to delay disease progression (Citation2,Citation9).
Diabetes
While preeclampsia is now recognized as a risk factor for cardiovascular disease, less attention has been given to the link between preeclampsia and the development of diabetes in the years after delivery. Chesley et al. highlighted an increased risk of late-onset diabetes in women with a history of eclampsia (Citation18). More recent studies have also suggested preeclampsia as a risk factor for developing diabetes (Citation19,Citation20). In a Canadian retrospective study, the risk of T2DM after a pregnancy complicated by hypertensive disorders (preeclampsia and gestational hypertension) was examined in a cohort of 1 million patients with a median follow-up of 8.5 years. This study showed that the risk of T2DM increases by 3.9% in women with pregnancy complicated by HT and by 6.6% in the case of PE alone (Citation20). Similarly, a Danish cohort study found a 3.12-fold (2.63–3.70) increased risk after a pregnancy complicated by gestational hypertension and a 3.68-fold (3.04–4.46) increase after severe preeclampsia (Citation21). However, the risk of developing diabetes was moderately higher in women with preeclampsia, especially when compared to women with gestational diabetes, who had a markedly elevated risk of developing it. On the other hand, when preeclampsia and gestational diabetes were associated, the probability was further higher (Citation22). To date, few studies have investigated the risk of developing type 1 and type 2 diabetes separately. A study by Savitz et al. reported a significantly higher risk of hospitalization for type 2 diabetes mellitus than for type 1 diabetes mellitus (aOR: 2.0, 95% CI: 1.3–3.2 vs aOR: 1.8, 95% CI: 0.8–3.8) (Citation23).
Cardiovascular risk
Several studies have highlighted how HDPs are associated with a greater cardiovascular risk. It is still unclear whether the cardiovascular changes associated with PE result in cardiovascular remodeling, increasing the lifetime risk of CVD, or whether PE is a manifestation of an underlying increased CV risk. For example, genetic and environmental factors such as hyperlipidemia, obesity, diabetes mellitus, or renal disease could predispose women to develop PE during pregnancy and CVD later in life (Citation5). Wu et al. reported a RR of all cardiovascular diseases resulting from HDP between 0.88 and 13.18, with a RR of 1.66 (95% CI 1.49–1.84) for coronary artery disease, 2.87 (95% CI 2.14–3.85) for heart failure, 1.60 (95% CI 2.14–3.85) for for peripheral vascular disease, and 1.72 (95% CI 1.50–1.97) for stroke (Citation24). This data was also confirmed in another meta-analysis from 2021 (Citation25) in which new interesting details emerged: women with early-onset PE (<34 weeks) were at increased risk of developing adverse cardiovascular outcomes, CVD, cerebrovascular disease, HT, dyslipidemia, renal dysfunction, and metabolic syndrome, compared with late-onset PE (Citation25). Previously, Bellamy et al. in a meta-analysis observed that women with early preeclampsia had the greatest risk of developing CVD in the future also compared to patients who had a severe form of PE (defined as a BP > 160/100 mmHg associated with proteinuria >0.3 g/24 h or diastolic BP >110 mmHg with proteinuria >5 gr/24 h). Moreover, a doubled risk of ischemic attack in women with prior preeclampsia (2.16, 1.86–2.52) was reported in the same study, with a greater risk of fatal ischemic attack in the case of severe PE (2.86, 2.25–3.65), compared to a moderate PE (1.92, 1.65–2.24) (Citation26). Similar findings emerged more recently, in a 2017 meta-analysis evaluating 2 studies with more than 250,000 women with PE. Patients who developed preeclampsia resulted in a 4-fold increased risk of heart failure after pregnancy. However, some confounding factors (obesity, diabetes, and smoking) influencing the association were reported (Citation6). Evaluating the risk of stroke after PE, the literature reports a risk of 1.81 (1.45–2.27), especially when the diagnosis of PE is done before 37th week (5.08, 2.09–12.35), compared with cases of PE after the 37th week (0.98, 0.50–1.92) (Citation26). Furthermore, there is a greater risk of fatal stroke.
Based on a study conducted by Veerneek et al., the number and severity of risk factors for postpartum CVD differ according to the type of disorder developed during pregnancy: early PE, late PE, or gestational HT (Citation27). Moreover, the authors conclude assuming that CVD risk after pregnancy may reflect CVD risk later in life, especially the risk of chronic hypertension. Indeed, women who had early PE showed the least favorable CV risk profile compared to those who had late PE or gestational HT, particularly for glucose and lipid levels. Postpartum HT was observed in approximately 50% of the patients with a history of PE and gestational HT. Furthermore, blood pressure values were significantly higher in women who had early PE and gestational HT compared with late PE.
Chronic hypertension
Women who develop HDP during pregnancy have a 2- to 8-fold increased lifetime risk of developing chronic hypertension compared with normotensive pregnant women (Citation2,Citation24,Citation26,Citation28,Citation29). However, the time of onset of HT after a pregnancy complicated by HDP remains unclear. The knowledge of the timing represents a crucial point because it would allow clinicians to plan a correct follow-up for these women, ensuring an early diagnosis of complications. A 2007 meta-analysis by Bellamy et al. (Citation26) including 3 million women between 1960 and 2006 found that 1885 of 3658 women whose pregnancy was complicated by PE, developed chronic HT during their lifetime (mean follow-up of 14.1 years). The relative risk of a subsequent diagnosis of HT was 3.70 (95% CI 2.70–5.05) for women who developed PE during pregnancy, compared to those who did not develop it. This risk was lower (2.37; 2.11–2.66) when considering only studies including larger numbers of women. In the same meta-analysis, authors also analyzed the correlation between the development of HT in pregnancy and the risk of developing chronic HT: the RR of chronic HT for women who had gestational HT compared to normotensive women was 3.39 (0.82–13.92). Recent studies have shown that the risk of developing HT after HDP already appears in the first months following pregnancy (Citation30–32). In a 2020 meta-analysis conducted by Giorgione et al. (Citation31), the risk of developing hypertension within 2 years of delivery in women whose pregnancy was complicated by HDP emerged to be 6 times greater than in normotensive women. Moreover, the risk was evaluated during three postpartum periods: within 6 months, between 6 months and 1 year and between 1 year and 2 years. The results reported an overall OR of 5.75 (95% CI 3.92–8.44). The OR rose to 13.39 (95% CI 1.27–141.04) in the period up to 6 months postpartum, decreased to 4.13 (95% CI 2.82–6.07) between 6 months and 1 year, and finally raised again to 8.73 (CI 95% 4.66–16.35) in the period between 1 and 2 years. The same analysis was done for pregnancy complicated by preeclampsia: women had a significantly increased risk of developing postpartum HT compared to women without PE (OR 6.83; 95% CI 4.25–10.96). Again, a discrepancy was observed in different periods: up to 6 months, OR 43.95 (95% CI. 5.72–338.04); between 6 months and 1 year, OR 4.46 (95% CI, 2.76–7.21); and between 1 and 2 years, OR 8.91 (95% CI 4.33–18.33). Thus, Giorgione et al. (Citation31) underlined the importance of the immediate postpartum period, a critical period that should be monitored closely. Finally, although the literature on this evidence is not yet clear, gestational HT more than PE was associated with chronic HT, in women older than 30 years (Citation24,Citation28).
Thromboembolic risk
Pregnancy increases the risk of thromboembolic events 5-fold compared to non-pregnant state, even in uncomplicated pregnancy (Citation29). The risk is higher in the case of preeclampsia, both during pregnancy and in the post-partum period.
A nationwide cohort study was conducted in the Netherlands to determine whether hypertensive pregnancy disorder is a risk factor for thromboembolic events both during pregnancy and in subsequent years. This study included about 2 million women for a median of 13.7 years (Citation33). The absolute risk after delivery in women with uncomplicated pregnancies was 2.1 (95% CI, 2.0–2.2) per 10 000 person years, 3.1 (95% CI, 2.9–3.3) per 10 000 person years in women with hypertension during pregnancy, and 4.3 (95% CI, 3.7–5.0) in women with pregnancy complicated by preeclampsia.
According to another Danish study, in which 1419 women with a past medical history of thromboembolic events were followed during their pregnancy, the risk of developing preeclampsia was higher in this group compared to women with a negative past medical history (RR 1.5 95% CI, 1.3–1.8) (Citation34). Therefore, this dual relationship suggests common physiopathological mechanisms, including endothelial dysfunction, platelet activation, and pro-inflammatory and procoagulant states (Citation35).
In a meta-analysis, Bellamy et al. (Citation26) included three studies analyzing the link between preeclampsia and subsequent risk of thromboembolic events, in a total of 35,772 women and a median of 4.7 years of follow-up. Women who developed preeclampsia during pregnancy, had an increased risk [1.79 (1.37–2.33)] of vascular disease compared with women without a diagnosis of PE. A further distinction was made by Kestenbaum et al. (Citation36). According to them, severe preeclampsia is associated with a higher risk of venous thromboembolism (2.3, 1.3–4.2) compared with moderate pre-eclampsia in years after delivery. Among thromboembolic complications, it is also important to keep in mind a less frequent but life-threatening event: the ovarian vein thrombosis. It occurs in 0.02–0.20% of all pregnancies and can be associated with HDP (Citation37,Citation38).
Follow up approach
The American Heart Association and the European Society of Cardiology have included hypertensive disorder of pregnancy within the main risk factor for cardiovascular disease (Citation28,Citation39). As also emerged in this review, these patients have a greater risk of developing cardiovascular, cerebrovascular, and arterial diseases and have a higher rate of mortality cardiovascular related (Citation40,Citation41). Scientific societies agree on the importance of follow-up on these patients (Citation3,Citation42–44). The ACOG guidelines suggest a checkup for women who have developed HDP after 7–10 days after childbirth and an assessment at 72 h in case of severe hypertension. Appropriate advice should be provided to explain the risk of developing cardiovascular disease in the future. Cardiovascular risk should be assessed individually to suggest appropriate lifestyle changes (Citation43). Moreover, women who developed pre-eclampsia in a past pregnancy should be advised that the rate of recurrence in subsequent pregnancies is around 15%, with a further risk of 15% to develop gestational hypertension (Citation44). A structured follow-up, including risk assessment, patient education, and lifestyle intervention should be considered as a standard of care for all women who had a hypertensive disorder of pregnancy. Early post-partum lifestyle intervention can prevent the development of cardiovascular disease and prevent morbidity and mortality (Citation3,Citation8).
The follow-up of these women should include (Citation3,Citation42,Citation43): 1. Visits with a family doctor to check up the blood pressure and lipid and glucose profiles; 2. Lifestyle changes (smoking cessation, maintaining ideal body weight, regular aerobic exercise, appropriate diet); 3. Eventually, start of a pharmacological therapy in case of persistent hypertension and/or dyslipidemia; 4. Eventual prophylaxis with low-dose aspirin in women with additional risk factors for stroke (Citation42,Citation45).
To reduce these risks in future pregnancies, it is useful to start low-dose aspirin prophylaxis between 12 weeks and 28 weeks of gestation (optimally before 16 weeks of gestation) and continuing until delivery (Citation3).
The ACOG guidelines suggest a checkup for women who developed HDP after 7–10 days post-partum and a 72 h evaluation in the case of severe hypertension.
A proper counseling should be offered to explain the risk of developing cardiovascular disease in the future. Cardiovascular risk should be evaluated individually to suggest appropriate lifestyle changes. The latest ACOG guidelines underline the importance of modifying lifestyle, to reduce possible CV risk factors and the need for long-term follow-up.
Pregnancy is an ideal time to maintain or start a healthy lifestyle. ACOG recommends that all specialists suggest adequate physical activity: several meta-analyses have demonstrated a significant reduction of HDP, gestational diabetes, and cesarean section in women who practice 30/60 min of aerobic exercise 2/7 times a week compared to sedentary women.
In addition, due to the benefits on the cardiovascular system and on the production of breast milk, an early resumption of physical activity is strongly encouraged during the puerperium, with a timing that depends on the mode of childbirth (Citation46).
Breastfeeding also has beneficial effects: the CARDIA study (Coronary Artery Risk Development in Young Adult) has demonstrated a protective connection between breastfeeding and metabolic syndrome after weaning. Other studies also seem to extend this protective effect to cardiovascular disease: among the participants to Nurses’ Health Study women who breastfed for 2 years had a 37% less chance of developing coronaropathy compared to women who did not breastfeed. These results confirm the importance of including this type of advice in the post-partum counseling of women whose pregnancy was complicated by HDP (Citation47).
Conclusions
Hypertensive disorders in pregnancy may be associated with immediate worsening of maternal–fetal outcomes, but the literature agrees that considering these pathologies is also a possible expression or cause of a greater cardiovascular risk in the long term in the life of the patient. The main complications include kidney injury, diabetes, and cardiovascular risks, such as chronic hypertension, stroke, myocardial infarction, and thromboembolism. Data are controversial about which HDP is associated with the worst outcomes and further research is certainly needed. The follow-up of these patients is becoming more and more important and is drawing the attention of clinicians. All medical visits, including the postpartum follow-up, should be considered an opportunity to focus on the importance of maintaining a healthy lifestyle and minimizing health risks. The gynecologist is a reference point in a woman’s life and must also take an active role in chronic disease prevention to improve women’s long-term health and quality of life (Citation48). This becomes even more important for those patients who have developed complications during pregnancy and who may present a higher risk.
Abbreviations
Disclosure statement
No potential competing interest was reported by the author.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardio- vascular risk factors in middle age: the Avon longitudinal study of parents and children. Circulation. 2012 Mar;125(11):1367–7.
- Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019 Aug;105(16):1273–1278.
- Gestational hypertension and preeclampsia: aCOG practice bulletin, number 222. Obstet Gynecol. 2020 Jun;135(6):e237–260. doi: 10.1097/AOG.0000000000003891
- Karrar SA, Hong PL Preeclampsia. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan [accessed 2023 Feb 13]. https://www.ncbi.nlm.nih.gov/books/NBK570611
- Ahmed R, Dunford J, Mehran R, et al. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–1822. doi: 10.1016/j.jacc.2014.02.529
- Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017 Feb;10(2):e003497.
- Arnott C, Nelson M, Alfaro Ramirez M, et al. Maternal cardiovascular risk after hypertensive disorder of pregnancy. Heart. 2020 Dec;106(24):1927–1933.
- Ackerman-Banks CM, Grechukhina O, Spatz E, et al. Seizing the window of opportunity within 1 year postpartum: early cardiovascular screening. J Am Heart Assoc. 2022 Apr 19;11(8):e024443. doi: 10.1161/JAHA.121.024443
- Kristensen JH, Basit S, Wohlfahrt J, et al. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019 Apr 29;365:l1516. doi: 10.1136/bmj.l1516
- Strevens H, Wide-Swensson D, Hansen A, et al. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG. 2003;110(9):831–836. doi: 10.1111/j.1471-0528.2003.02162.x
- Henao DE, Saleem MA. Proteinuria in preeclampsia from a podocyte injury perspective. Curr Hypertens Rep. 2013;15(6):600–605.
- Craici IM, Wagner SJ, Weissgerber TL, et al. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014;86(2):275–285. doi: 10.1038/ki.2014.17
- McDonald SD, Han Z, Walsh MW, et al. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010;55(6):1026–1039. doi: 10.1053/j.ajkd.2009.12.036
- Wang IK, Muo CH, Chang YC, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;185(3):207–213. doi: 10.1503/cmaj.120230
- Wu CC, Chen SH, Ho CH, et al. End-stage renal disease after hypertensive disorders in pregnancy. Am J Obstet Gynecol. 2014;210(2):147. doi: 10.1016/j.ajog.2013.09.027
- Ayansina D, Black C, Hall SJ, et al. Long term effects of gestational hypertension and pre-eclampsia on kidney function: record linkage study. Pregnancy Hypertens. 2016;6(4):344–349. doi: 10.1016/j.preghy.2016.08.231
- Dai L, Chen Y, Sun W, et al. Association between hypertensive disorders during pregnancy and the subsequent risk of end-stage renal disease: a population-based follow-up study. J Obstet Gynaecol Can. 2018 Sep [2018 Jun 19];40(9):1129–1138. doi: 10.1016/j.jogc.2018.01.022
- Chesley LC. Remote prognosis after eclampsia. Perspect Nephrol Hypertens. 1976;5:31–40.
- Feig DS, Shah BR, Lipscombe LL, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. 2013 Apr 16;10(4):e1001425. doi: 10.1371/journal.pmed.1001425
- Libby G, Murphy DJ, McEwan NF, et al. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: an intergenerational study from the Walker cohort. Diabetologia. 2007; 50(3):523–530. doi: 10.1007/s00125-006-0558-z
- Lykke JA, Langhoff-Roos J, Sibai BM, et al. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009 Jun Epub 2009 May 11;53(6):944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765
- Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015 Mar;15(3):9. doi: 10.1007/s11892-015-0579-4
- Savitz DA, Danilack VA, Elston B, et al. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180(1):4–14. doi:10.1093/aje/kwu118
- Wu R, Wang T, Gu R, et al. Hypertensive disorders of pregnancy and risk of cardiovascular disease-related morbidity and mortality: a systematic review and meta-analysis. Cardiol. 2020 Epub 2020 Aug 25;145(10):633–647. doi: 10.1159/000508036
- Dall’asta A, D’Antonio F, Saccone G, et al. Cardiovascular events following pregnancy complicated by pre-eclampsia with emphasis on comparison between early- and late-onset forms: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021 May;57(5):698–709. doi: 10.1002/uog.22107
- Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007 Nov 10 [2007 Nov 1];335(7627):974. doi: 10.1136/bmj.39335.385301.BE
- Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015 Mar [2015 Jan 5];65(3):600–606. doi: 10.1161/HYPERTENSIONAHA.114.04850
- Mosca L, Benjamin EJ, Berra K, et al. American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011 Mar 22;57(12):1404–1423. Erratum in: J Am Coll Cardiol. 2012 May 1;59(18):1663. doi: 10.1016/j.jacc.2011.02.005
- James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194(5):1311–1315. doi: 10.1016/j.ajog.2005.11.008
- Behrens I, Basit S, Melbye M, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017 Jul 12;358:j3078. doi: 10.1136/bmj.j3078
- Giorgione V, Ridder A, Kalafat E, et al. Incidence of postpartum hypertension within 2 years of a pregnancy complicated by pre-eclampsia: a systematic review and meta-analysis. BJOG. 2021 Feb;128(3):495–503. doi: 10.1111/1471-0528.16545. Epub 2020 Oct 21.
- Benschop L, Duvekot JJ, Versmissen J, et al. Blood pressure profile 1 year after severe preeclampsia. Hypertension. 2018 Mar;71(3):491–498.
- Scheres LJJ, Lijfering WM, Groenewegen NFM, et al. Hypertensive Complications of Pregnancy and Risk of Venous Thromboembolism. Hypertension. 2020 Mar [2020 Jan 13];75(3):781–787. doi: 10.1161/HYPERTENSIONAHA.119.14280
- Hansen AT, Schmidt M, Horváth-Puhó E, et al. Preconception venous thromboembolism and placenta mediated pregnancy complications. J Thromb Haemost. 2015;13(9):1635–1641. doi: 10.1111/jth.13046
- Egan K, Kevane B, Áinle F N. Elevated venous thromboembolism risk in preeclampsia: molecular mechanisms and clinical impact. Biochem Soc Trans. 2015;43(4):696–701.
- Kestenbaum B, Seliger SL, Easterling TR, et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003 Nov;42(5):982–989. doi: 10.1016/j.ajkd.2003.07.001
- Angelini M, Barillari G, Londero AP, et al. Puerperal ovarian vein thrombosis: two case reports. J Thromb Thrombolysis. 2013 Feb;35(2):286–289. doi: 10.1007/s11239-012-0794-7
- Bukhari S, Fatima S, Barakat AF, et al. Venous thromboembolism during pregnancy and postpartum period. Eur J Intern Med. 2022 Mar Epub 2021 Dec 20;97:8–17. doi: 10.1016/j.ejim.2021.12.013
- Authors/Task Force MembersPiepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016 Jul [2016 Jun 27];23(11):NP1–96. doi: 10.1177/2047487316653709
- Grandi SM, Filion KB, Yoon S, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019 Feb 19;139(8):1069–1079. Erratum in: Circulation. 2019 Aug 27;140(9):e544. doi: 10.1161/CIRCULATIONAHA.118.036748
- Ray JG, Vermeulen MJ, Schull MJ, et al. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005 Nov 19;366(9499):1797–1803. doi: 10.1016/S0140-6736(05)67726-4
- Italian Preeclampsia Association. [Hypertensive disorders in pregnancy: classification, diagnosis and therapy. Recommendations of good clinical practice AIPE]. Italian 2020. https://www.sigo.it/wp-content/uploads/2020/11/RaccomandazioniAIPEDisordini_Ipertensivi_Gravidanza.pdf
- ACOG Committee Opinion No. 736: optimizing Postpartum Care. Obstet Gynecol. 2018 May;131(5):e140–150. doi: 10.1097/AOG.0000000000002633
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management (NICE guideline NG133). Published: 25 Jun 2019. https://www.nice.org.uk/guidance/ng133
- Bushnell C, McCullough LD, Awad IA, et al., American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council for High Blood Pressure Research. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–1588. Medline:24503673. doi: 10.1161/01.str.0000442009.06663.48
- ACOG Committee Opinion No. 804: physical activity and exercise during pregnancy and the postpartum period: correction. Obstet Gynecol. 2021 Oct 1;138(4):683. doi: 10.1097/AOG.0000000000004558
- Graves M, Howse K, Pudwell J, et al. Pregnancy-related cardiovascular risk indicators: primary care approach to postpartum management and prevention of future disease. Can Fam Physician. 2019 Dec;65(12):883–889.
- Brown HL, Warner JJ, Gianos E, et al. American Heart Association and the American College of Obstetricians and Gynecologists. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018 Jun 12Epub 2018 Jun 12;137(24):e843–852. doi: 10.1161/CIR.0000000000000582

