ABSTRACT
Objective
Estimate the prevalence of hypertensive disorder of pregnancy (HDP) at term, define population characteristics, and calculate adverse maternal outcomes.
Methods
Retrospective study.
Results
We included 4,702,468 pregnancies. HDP increased linearly from 4.5% (2014) to 6.0% (2018). HDP was more frequent among black (PR 1.19), obese (PR 2.31 to 3.70), with gestational (PR 1.87) or pregestational diabetes (PR 2.16). Increased transfusion (PR 2.52), intensive care unit admission (PR 3.38), and unplanned hysterectomy (PR 1.78) with HDP.
Conclusion
Our study quantifies the increased risks for maternal and neonatal complications related to the development of HDP at or beyond 39 weeks among nulliparous women.
Introduction
Individuals expectantly managed beyond 39 weeks of gestation are considered to be at lower risk for adverse maternal and neonatal outcomes. Risk factors identified during pregnancy would most often require intervention and delivery by this stage of pregnancy. For these individuals, the optimal timing for delivery is debated. With the ARRIVE trial, there are compelling data for the safety of induction of labor (IOL) at 39 weeks for low-risk nulliparous individuals without increasing complication rates (Citation1). That study determined that there are maternal risks associated with remaining pregnant beyond 39 weeks of gestation. Notably, in this randomized control trial that looked at IOL versus expectant management of low-risk individuals after 39 weeks, individuals who underwent IOL had a decreased risk of hypertensive disorder of pregnancy (HDP) (9.1% vs. 14.1%; relative risk, 0.64; 95% confidence interval (CI), 0.56–0.74) (Citation1). This is further supported by Redman et al., who demonstrated that with increasing gestational age, metabolic factors representative of syncytiotrophoblast stress start to trend similarly to those that are seen in early HDP. With ongoing placental maturation and increased gestational age, HDP may be inevitable (Citation2–4); however, this increased risk is not reliably predicted (Citation5).
These and other similar studies (Citation6,Citation7) suggest an increased risk of developing HDP in an otherwise lower-risk pregnancy with continuing gestation after 39 weeks, but the magnitude of this risk is not well defined. For counseling, the clinician would benefit from knowing what the harms of expectant management after 39 weeks are regarding the development of HDP and its adverse maternal and neonatal outcomes. Previous studies have shown increased rates of maternal morbidity such as intensive care unit (ICU) admission, unplanned hysterectomy, blood transfusion, or uterine rupture with advancing gestation after 39 weeks in low-risk nulliparous individuals (Citation8,Citation9). However, these studies have excluded individuals with hypertensive diagnoses in their analysis, therefore removing any additional risk attributed to the development of HDP after 39 weeks.
Using population-based data from deliveries in the United States, the aims of our study are to (1) estimate the prevalence of HDP after 39 weeks of gestation, (2) identify maternal and delivery factors associated with the development of HDP in this period, and (3) examine the relationship between HDP and adverse maternal outcomes. We hypothesize that nulliparous individuals, who develop HDP after 39 weeks, will have greater rates of maternal adverse outcomes than those who do not.
Material and methods
This was a population-based retrospective cohort study using live birth databases from the U.S. National Center for Health Statistics (Citation10). The National Vital Statistics data set is compiled from data reported on all birth certificates shortly after the live birth of an infant including parental demographic information, maternal reproductive and pregnancy history, medical procedures, and infant birth weight. It is collected from all 50 states and the District of Columbia and New York City and makes up more than 99% of all births for a given year. Because the datasets are publicly available and do not contain direct personal identifiers, this study was considered exempt from review by the institutional review board at the University of Minnesota (IRB exemption STUDY00007628). Our study sample was restricted to births to nulliparous individuals who delivered between 2014 and 2018 with a singleton, cephalic presenting, non-anomalous fetus, who experienced spontaneous onset of labor and delivered at 39, 40, 41, or 42 weeks of gestation. A reference group of births at 36–38 weeks of gestation was included to establish a perspective of the rate of HDP ≥39 weeks of gestation. For purposes of this study, we only included pregnancies having either no significant medical comorbid conditions or well-controlled comorbid conditions that do not mandate urgent IOL. In accordance with this definition, we did include births complicated by pregestational diabetes, gestational diabetes, or chronic hypertension as these conditions, if well-controlled, are managed expectantly past 38 6/7 weeks and consistent with current guidelines (Citation11).
Our outcome variable was the diagnosis of HDP, documented in the dataset as either gestational hypertension or hypertension-eclampsia. We selected delivery characteristics including calendar year, gestational age (recorded in full weeks), IOL (yes, no), mode of delivery, birthweight, and complications at or after delivery. We also selected maternal demographic and health characteristics: age, race/ethnicity, nativity, educational attainment, body mass index (BMI), smoking status pre-pregnancy, smoking status during pregnancy, and variables from the maternal risk factor series (pre-pregnancy diabetes, gestational diabetes, and pre-pregnancy hypertension). Complications comprised maternal transfusion, ICU admission, unplanned hysterectomy, or a composite of any of the above. We chose these morbidities as they represent significant clinical outcomes in pregnancy, are complications associated with HDP, and available in the birth record data (Citation10).
In preparation for analysis, we performed a secondary exclusion of records found to be missing data on the entire maternal risk factor series (including hypertension and diabetes); most of these records were from 2014 and 2015. We examined the distributions of all variables in the sample with univariate descriptive statistics. Cross-tabulations were used to obtain the crude prevalence of HDP by year, gestational week of delivery, delivery characteristics, and maternal characteristics. Using modified Poisson regression with robust standard errors, we also estimated prevalence ratios (PRs) with 95% CIs to examine the relative association between all covariates and HDP (Citation12). Models for maternal characteristics were adjusted for age, race/ethnicity, and nativity; adjustment for remaining covariates could have yielded biased estimates as temporal relationships could not be established for this cross-sectional dataset. Crude prevalence and PRs were also computed for complications but with HDP as the exposure and complications as outcomes. For analyses by gestational week of delivery only, we included data from births at 36–38 weeks meeting the same eligibility criteria as our main sample. This reference group was chosen to contextualize our findings regarding the incidence of HDP for deliveries at 39–42 weeks, as most individuals diagnosed with preterm HDP without severe features are delivered at 37 weeks (Citation13). We performed sensitivity analysis for the PR of HDP as well as for the maternal complications reported by excluding all pregnancies complicated by pregestational diabetes or hypertension. Analyses were performed in Stata v14.2 (StataCorp, College Station, Texas, USA) using raw data files formatted for Stata by the National Bureau of Economic Research (Citation14).
Results
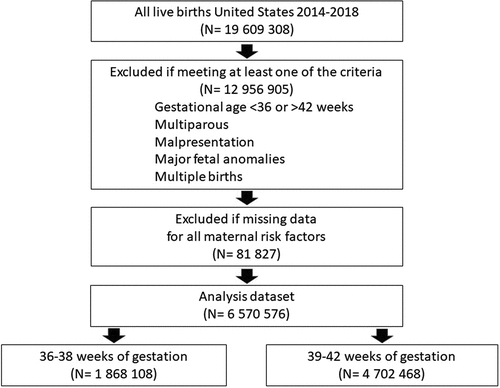
From 2014 to 2018, there were 19,609,308 live births recorded (). After exclusion criteria, 4,702,468 individuals were included in our main analysis and an additional 1,868,108 individuals contributed data to our analysis by gestational week. The prevalence of HDP increased linearly through the study period from 4.5% in 2014 to 6.0% in 2018 or approximately 7% per year (PR 1.07, 95% CI 1.07–1.08).
We observed a J-shaped pattern in the prevalence of HDP by gestational week ( and ). The prevalence was highest at 37 weeks (16.7%), decreased to a low of 3.8% at 41 weeks, and rebounded slightly at 42 weeks (5.0%). Findings for gestational weeks 39–42 are further described in , where the study cohort is depicted by week of gestation, and the proportion that developed preeclampsia stratified by IOL status. One-third of individuals underwent IOL (33.1%), of whom 9.9% were diagnosed with HDP vs. 2.9% among individuals without IOL (data not shown). For each gestational age, patients who underwent an IOL had two to four times higher rates of HDP than individuals who did not undergo IOL. The highest prevalence of HDP was in the subset of individuals at 39 weeks who underwent an IOL. These findings were not modified after sensitivity analysis (Table S1 supplement).
Figure 2. The proportion of study cohort in each week of gestation and prevalence of hypertensive disorders in pregnancy according to the induction of labor status. HDP: hypertensive disorders in pregnancy.
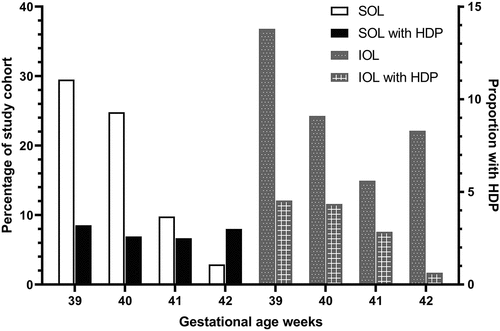
Table 1. Unadjusted prevalence ratios of hypertensive disorders in pregnancy by gestational age.
Most newborns had a birthweight >2500 g (98.4%). The incidence of low birthweight (1500–2500 g) and very low birthweight (<1500 g) was 1.6% and 0.04%, respectively. The prevalence of HDP increased with decreasing birthweight. Among low-birthweight newborns, 11.5% had HDP (PR 2.25, 95% CI 2.2–2.3), and among very-low-birthweight newborns, 18.8% had HDP (PR 3.67, 95% CI 3.33–4.06). Cesarean delivery occurred in 28.7% of the population, and among these individuals, the PR for HDP was 1.56 (95% CI 1.55–1.57) compared with those who had a vaginal delivery.
The prevalence of HDP also varied by maternal characteristics (). Compared to people aged 25–29 at delivery, the prevalence was 9–10% lower among individuals younger than 20 and significantly higher for individuals aged 35–39 (adjusted prevalence ratio (aPR) 1.09, 95% CI 1.08–1.11), 40–44 (aPR 1.24, 95% CI 1.20–1.28), or older (aPR 1.56, 95% CI 1.42–1.72). Compared with white individuals, American Indian or Alaska Native individuals and Black individuals had a significantly higher prevalence (aPR 1.28, 95% CI 1.23–1.34 and 1.19, 95% CI 1.17–1.20, respectively) while Asian and Hispanic individuals were significantly less likely to have HDP (aPR 0.62, 95% CI 0.61–0.64 and 0.90, 95% CI 0.89–0.91, respectively). The prevalence was also higher among individuals whose educational attainment was equal or less than college vs. higher (aPR range 1.11–1.39) and who were overweight (aPR 1.64, 95% CI 1.63–1.66) or obese (aPR range 2.30–3.70) vs. normal weight, smoked before (aPR 1.09, 95% CI 1.07–1.10) pregnancy, or had pregestational (aPR 2.16, 95% CI 2.07–2.24) or gestational diabetes (aPR 1.87, 95% CI 1.84–1.90). Individuals born outside the U.S. had 29% lower prevalence of HDP. Pregestational hypertension also appeared to be associated with a lower risk (PR 0.10, 95% CI 0.09–0.11), although we believe this to be an artifact of our study selection criteria given since most guidelines recommend that individuals with known HDP or unstable or severe chronic hypertension be delivered prior to 39 weeks.
Table 2. Maternal characteristics and prevalence of hypertensive disorders in pregnancy.
Individuals diagnosed with HDP were more likely to experience maternal complications such as transfusion, ICU admission, and unplanned hysterectomy, as well as a composite of all these outcomes. Transfusion occurred in only 0.3% of our sample but was nearly three times more common among individuals with HDP (PR 2.73 95% CI 2.60–2.87). ICU admission and unplanned hysterectomy occurred even less frequently but were likewise positively associated with HDP (PR 3.89, 95% CI 3.59–4.20 and 1.86, 95% CI 1.45–2.40, respectively). Conversely, HDP occurred three times more often among individuals with any of the reported maternal complications (PR 2.73, 95% CI 2.63–2.83, ). The overall findings did not change after excluding all cases of pregestational diabetes or hypertension (Table S2 supplement).
Table 3. Prevalence of hypertensive disorders in pregnancy as outcome in patients with maternal morbidities.
Discussion
Our primary objective to quantify the prevalence of HDP among lower-risk women after 39 weeks of gestation found an increasing prevalence between 2014 and 2018 with a 7% annual increase. We found that in this national birth cohort, women who delivered at 39 weeks had the highest prevalence of HDP (6.3%) with decreasing prevalence from 39 to 41 weeks and an increased prevalence at 42 weeks. Overall, the prevalence of HDP after 39 weeks was notably decreased compared with deliveries between 36 and 38 weeks of gestation due to higher rates of IOL secondary to the development of preterm HDP as well as to delivery of women with unstable or severe pregestational hypertension (Citation11,Citation15). In this cohort, ongoing pregnancies with chronic hypertension would most likely be women with a diagnosis of chronic hypertension but with stable blood pressure that did not require medication or who declined IOL. Our study demonstrated that while the prevalence of HDP is decreased for lower populations who deliver between 39 and 42 weeks, the residual risk is approximately 1 in 20.
We confirmed that maternal characteristics that are associated with a higher prevalence of HDP after 39 weeks include pregestational and gestational diabetes, BMI > 25, and advanced maternal age, which is consistent with previously identified risk factors (Citation13). Preeclampsia was also slightly more prevalent in women who smoked pre-pregnancy (aPR 1.09, 95% CI 1.07–1.10), while our estimate for smoking during pregnancy included the null. This is inconsistent with a large contemporary meta-analysis which showed that smoking during pregnancy was protective from preeclampsia (relative risk of 0.67, 0.60–0.75); the authors acknowledged that additional prospective studies were needed (Citation16). It has been recently demonstrated that cigarette smoking can enhance placental growth factor expression in early stages of trophoblast invasion (Citation17), while in the term placenta markers, smoking leads to increased markers of placental stress (Citation18,Citation19). Due to known differences between early- and late-onset preeclampsia, the effect of smoking may differ depending on the gestational age at onset of disease with a decreased risk for early-onset disease and increased risk for late-onset disease.
Women with pregestational hypertension (1%) were a small portion of our sample and had a decreased prevalence of HDP, 0.6%. Pregestational hypertension is a known risk factor for HDP and so one would assume an increased prevalence in this population (Citation13). It is possible that those individuals with pregestational hypertensive had well-controlled blood pressures and that would be consistent with ongoing management past 37 weeks. If medication masked a rise in blood pressure potentially associated with HDP, because of the nature of the disease, they would have other signs, symptoms, or laboratory changes not affected by medication. However, our findings of a decreased prevalence of HDP in patients with pregestational hypertension are most likely due to the gestational age limits of our sample. As current ACOG guidelines recommend delivery for women with poorly controlled pregestational hypertension prior to 39 weeks, we would expect women with more severe disease to not be included in our study population (Citation13). Women with poorly controlled pregestational hypertension are at increased risk of developing superimposed HDP and preterm delivery compared with women without pregestational hypertension and thus are less likely to be present in our study population (Citation20,Citation21). We did not exclude women with pregestational hypertension from our study, as our goal was to find women with increased risk of HDP who remain pregnant after 38 weeks. ACOG guidelines also allow for expectant management of women with pregestational hypertension that is well controlled with or without medications up to 39 weeks of gestation (Citation21).
Our third objective was to identify maternal morbidity associated with HDP. Our results support our hypothesis that patients with HDP would have an increased rate of maternal complications including increased rates of blood transfusion, ICU admission, and unplanned hysterectomy. As would be expected in a lower-risk population, the absolute risk of significant maternal morbidity was low, less than 1% each, for transfusion, ICU admission, or unplanned hysterectomy. These outcomes were found to be more prevalent among women with HDP. The increased prevalence of these complications in the population with HDP may be secondary to the increased risk of postpartum hemorrhage with HDP as well as increased risk of ICU admission for postpartum patients with HDP (Citation22–24).
While HDP is a causal factor for a proportion of maternal morbidity, increasing gestation also has been shown to increase maternal morbidity. In a study assessing maternal and neonatal morbidity between 39 and 41 weeks in labored women using US Vital Statistics data, Chen et al. found that increasing gestation after 39 weeks was associated with increased rates of a composite of maternal morbidity including transfusion, ICU admission, uterine rupture, and unplanned hysterectomy (Citation8,Citation9). Women with HDP were excluded from this study and any additional risk attributed to HDP would not be included. It can be assumed that a fraction of morbidity found in our study is due to advancing gestation alone; however, the finding of an increased maternal morbidity among individuals with HDP highlights the additional accumulated risks from HDP with advancing gestation over 39 weeks.
We have compared our results with those reported in the ARRIVE trial (Citation1) designed to compare labor induction with expectant management after 39 weeks. This trial reported HDP as a secondary outcome without additional information on the population regarding the prevalence by week or its association with other maternal morbidity. A secondary analysis of this trial observed an increase in the frequency of medically indicated IOL and cesarean delivery but not powered to measure the outcomes we have reported (Citation25). Compared with the ARRIVE trial, in which the prevalence of HDP was 11.5% in both IOL and expectant management groups combined, we found the prevalence of HDP to be less than half of that, at 5.2%. Factors that may have influenced this difference include ethnicity and BMI. More than half of the population studied in the ARRIVE trial had a BMI ≥30, and only 21% of our sample had a BMI ≥30. The ARRIVE trial also had increased rates of women of black non-Hispanic ethnicity, 23.1 vs. 11.9%, which has also been reported to be associated with increased rates of HDP (Citation26). Additionally, our reported prevalence may have been lower due to underreporting in the birth certificate data.
Our study did not exclude women with diabetes or pregestational hypertension. Including patients with these comorbidities likely contributed to the increased maternal morbidity, as some women with these comorbidities are likely to have escaped earlier delivery perhaps by patient preference or clinical nuance. However, our goal was to include women who may have had well-controlled disease, and therefore, clinical management up to 39 weeks and 6 days was a reasonable option. We cannot exclude the possibility that women with poorly controlled disease were included but believe that they would have made up a small portion of our sample due to current practice guidelines (Citation11,Citation27,Citation28). Future research should lead to a better understanding of the relationship between HDP and maternal morbidity, and how prevention of HDP could modify the risk for other adverse outcomes.
The strengths of this study include a population-based design with a large sample over 5 years. The sample population encompassed a large portion of deliveries within the United States and so is likely reflective of nulliparous women between 39 and 42 weeks gestation. Our sample size provides us with sufficient power to discern differences in uncommon although clinically important outcomes. Other strengths are the chosen maternal complications reflecting significant clinical outcomes that are easily measurable. Despite these outcomes being rare, lower risk nulliparous women make up a significant proportion of deliveries, and despite their low frequency, it will lead to a significant morbid condition in a significant number of women.
The largest study limitation is that it is a population retrospective study; thus, clinical intent cannot be interpreted, and a variety of clinical information is unavailable. For example, the indication for IOL is not available and so timing of development of HDP relative to decision to deliver cannot be obtained. The prevalence of HDP was more than 2–4 times greater in women undergoing IOL, but it cannot be ascertained whether HDP was the indication for induction or simply diagnosed during the admission (Citation29). Additionally, some conditions such as postpartum HDP may be underreported, as it may have been a second admission or late postpartum after the data for the birth certificate had been collected for a patient (Citation30). However, these potentially missing data points would likely affect the maternal morbidity outcomes if women were admitted to the ICU after a readmission. The third limitation is that birth certificates provide outcome information that we have reported but do not provide any information on the indication or reason for these outcomes such as ICU admission, unplanned hysterectomy, or transfusion. The only information is the association of HDP and postpartum hemorrhage (Citation23), which is associated with a higher transfusion rate.
Conclusions
While there is increasing evidence for the safety of IOL at 39 weeks in nulliparous women with lower risk pregnancies, the potential risk of development of HDP for the remaining pregnant individuals had not been quantified in a large population-based study. Our study demonstrates the maternal risks of expectant management after 39 weeks of gestation by demonstrating and quantifying the increased risks attributed to the development of HDP after 39 weeks among nulliparous women.
Authorship
All authors participated in the preparation of this manuscript in the following ways: conception and/or design of the work that led to the submission, data acquisition, and result interpretation. All have helped in drafting and reviewing the manuscript and have approved the final version. Finally, all have agreed to be accountable for all aspects of the work in ensuring that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental Material
Download Zip (27.8 KB)Acknowledgments
This project has been carried out with support from the Department of Obstetrics and Gynecology. We would like to acknowledge Erin Zielinski and Lauren Asfaw for their administrative support in carrying out and completing this project and manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
This is publicly available data that can be accessed at: http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2217452.
Additional information
Funding
References
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379(6):513–9. DOI:10.1056/NEJMoa1800566
- Redman EK, Hauspurg A, Hubel CA, et al. Clinical course, associated factors, and blood pressure profile of delayed-onset postpartum preeclampsia. Obstet Gynecol. 2019;134(5):995–1001.
- Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213(4 Suppl):S9.e1, S9–11.
- Wright D, Wright A, Nicolaides KH. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol. 2020;223(1):12–23.e7.
- De Kat AC, Hirst J, Woodward M, et al. Prediction models for preeclampsia: a systematic review. Pregnancy Hypertens. 2019;16:48–66.
- Gibson KS, Waters TP, Bailit JL. A risk of waiting: the weekly incidence of hypertensive disorders and associated maternal and neonatal morbidity in low-risk term pregnancies. Am J Obstet Gynecol. 2016;214(3):389e1–e12. doi:10.1016/j.ajog.2015.09.095
- Caughey AB, Stotland NE, Escobar GJ. What is the best measure of maternal complications of term pregnancy: ongoing pregnancies or pregnancies delivered? Am J Obstet Gynecol. 2003;189(4):1047–1052.
- Chen HY, Grobman WA, Blackwell SC, et al. Neonatal and maternal adverse outcomes among low-risk parous women at 39-41 weeks of gestation. Obstet Gynecol. 2019;134(2):288–294.
- Chen HY, Grobman WA, Blackwell SC, et al. Neonatal and maternal morbidity among low-risk nulliparous women at 39–41 weeks of gestation. Obstet & Gynecol. 2019;133(4):729–737.
- National Center for Health Statistics. Natality Data accessed 2022 Nov 21: http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm
- American College of Obstetricians and Gynecologists. Medically indicated late-preterm and early-term deliveries: ACOG committee opinion, number 831. Obstet Gynecol. 2021;138(1):e35–9. DOI:10.1097/AOG.0000000000004447.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706.
- American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135(6):e237–60. DOI:10.1097/AOG.0000000000003891.
- National Bureau of Economic Research. 2022 accessed 2022 Nov 21: https://www.nber.org/research/data/vital-statistics-natality-birth-data.
- American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia: ACOG practice bulletin summary, number 222. Obstet Gynecol. 2020;135(6):1492–1495. DOI:10.1097/AOG.0000000000003892.
- Wei J, Liu CX, Gong TT, et al. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget. 2015;6(41):43667–43678.
- Kawashima A, Koide K, Hasegawa J, et al. Maternal smoking history enhances the expression of placental growth factor in invasive trophoblasts at early gestation despite cessation of smoking. PLoS ONE. 2015;10(7):e0134181. DOI:10.1371/journal.pone.0134181
- Mitlid-Mork B, Bowe S, Gran JM, et al. Maternal placental growth factor and soluble fms-like tyrosine kinase-1 reference ranges in post-term pregnancies: a prospective observational study. PLoS ONE. 2020;15(10):e0240473. DOI:10.1371/journal.pone.0240473
- Mitlid-Mork B, Bowe S, Staff AC, et al. Alterations in maternal sFlt-1 and PlGF: time to labor onset in term-/late-term pregnancies with and without placental dysfunction. Pregnancy Hypertens. 2022;30:148–153.
- Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26–50. DOI:10.1097/AOG.0000000000003020.
- Oud L. Epidemiology of pregnancy-associated ICU utilization in Texas: 2001 - 2010. J Clin Med Res. 2017;9(2):143–153.
- Nyfløt LT, Sandven I, Stray-Pedersen B, et al. Risk factors for severe postpartum hemorrhage: a case-control study. BMC Pregnancy Childbirth. 2017;17(1):17. DOI:10.1186/s12884-016-1217-0
- Wetta LA, Szychowski JM, Seals S, et al. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol. 2013;209(1):51.e1–6.
- Tita ATN, Doherty L, Grobman WA, et al. Maternal and perinatal outcomes of expectant management of full-term, low-risk, nulliparous patients. Obstet & Gynecol. Feb 1 2021;137(2):250–257. DOI:10.1097/AOG.0000000000004230
- Caughey AB, Stotland NE, Washington AE, et al. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet Gynecol. 2005;106(1):156–161.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–64. DOI:10.1097/AOG.0000000000002501.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No.201: pregestational diabetes mellitus. Obstet Gynecol. 2018;132(6):e228–48. DOI:10.1097/AOG.0000000000002960.
- Roberts CL, Bell JC, Ford JB, et al. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy. 2008;27(3):285–297.
- Schoendorf KC, Branum AM. The use of United States Vital Statistics in perinatal and obstetric research. Am J Obstet Gynecol. 2006;194(4):911–915.