ABSTRACT
Objective
Investigate how hypertension during pregnancy (HDP) and depression during pregnancy (DDP) independently and jointly affect infant birth outcomes.
Methods
This population-based, retrospective cohort study included a sample of 68,052 women who participated in PRAMS 2016–2018 survey. Poisson regression was used for adjusted relative risks (aRRs).
Results
Compared to women without HDP and DDP, aRRs for PTB and LBW among women with both HDP and DDP are 2.04 (95% CI 1.73, 2.42) and 2.84 (95% CI 2.27, 3.56), respectively, albeit lower than the expected joint effect of risk.
Conclusion
DDP may modify the association between HDP and PTB, LBW.
Introduction
Hypertensive disorders of pregnancy (HDP), including chronic hypertension, gestational hypertension, pre-eclampsia/eclampsia, and chronic hypertension with superimposed pre-eclampsia, occur in approximately 5% to 10% of pregnancies (Citation1). It accounts for nearly 16% of maternal deaths and is considered the single leading cause of maternal mortality in industrialized countries (Citation2). Numerous studies have linked HDP with adverse birth outcomes such as preterm birth (PTB), low birth weight (LBW), and small-for-gestational-age (SGA) (Citation3–5). The effects of HDP on birth outcomes were more evident from the second to third trimester of pregnancy (Citation6–9), and women with pre-eclampsia or eclampsia usually had highest risk of PTB and LBW compared to women with other types of HDP (Citation10–12). Furthermore, some studies found pre-eclampsia was associated with large-for-gestational-age (LGA), although the direction of the association was inconsistent (Citation13,Citation14).
Recent research also recognized the importance of depression during pregnancy (DDP) as a risk factor associated with adverse birth outcomes (Citation15,Citation16). Major depressive disorder complicates up to 12.7% of pregnancies and as many as 37% of women have at least some depressive symptoms during their pregnancies (Citation17,Citation18). However, evidence on the association between DDP and adverse birth outcomes is inconclusive (Citation17,Citation19,Citation20). Mixed results were also observed regarding the impact of DDP on PTB and SGA across different racial/ethnic subgroups (Citation21,Citation22), but few studies investigated the association between depression and LGA (Citation23,Citation24).
Despite the cumulative evidence supporting the independent effect of HDP and DDP on birth outcomes, the joint effect of HDP and DDP has rarely been studied. Few existing studies investigating such joint effect on birth outcomes are mostly based on small sample sizes and address the effect of other types of psychological problems (e.g., stress, anxiety) on birth outcomes (Citation25,Citation26). The aims of the present study were to examine 1) the independent association between HDP or DDP and LBW, PTB, SGA, and LGA; and 2) whether DDP modifies the association between HDP and birth outcomes in a large, U.S. population-based cohort study. We hypothesized that HDP and DDP would independently and jointly increase the risk of adverse birth outcomes.
Materials and methods
Study population
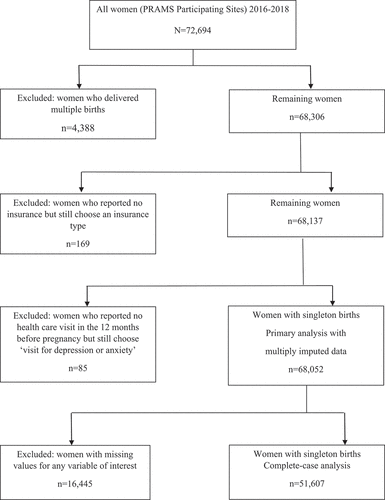
We conducted a population-based, retrospective cohort study on the effect of HDP and DDP on birth outcomes. This study was based on data from the Phase 8 (2016–2018) survey of Pregnancy Risk Assessment Monitoring System (PRAMS). The Centers for Disease Control and Prevention (CDC) and state health departments jointly developed the PRAMS to identify high-risk women and infants and monitor their health status (Citation27). PRAMS is currently used to assess about 83% of all U.S. births and collects information on maternal characteristics, behaviors, and experiences prior to conception, during pregnancy, and after delivery (Citation27). Data are collected from a selected sample of women who had a recent live birth from each state by mail first and subsequently by a telephone interview if there is no response to repeated mailings. PRAMS data are also linked with birth certificate data which provide additional demographic and medical information for analysis (Citation27). All women who completed the Phase 8 questionnaire (n = 72,694) with singleton births were eligible for the present study (n = 68,306). After excluding women who did not answer questions in the questionnaire with skip patterns appropriately for the insurance (n = 169) and healthcare visit variables (n = 85), our primary analytical sample included 68,052 women with 16,445 of them having missing data that were imputed for analysis ().
Outcomes
Our outcomes of interest include PTB, LBW, SGA, and LGA. The World Health Organization (WHO) defines PTB as all births delivered at less than 37 weeks of completed gestation, and LBW as birth weights less than 2500 g (Citation28). SGA and LGA were determined in the birth certificate based on 10th percentile and below or 90th percentile and above for gestational age and weight, respectively.
Exposure
The primary exposure in this study was HDP, and the potential effect modifier was DDP. These two variables were assessed in the PRAMS questionnaire through the question “During your most recent pregnancy, did you have any of the following health conditions?” (Citation29) Women who checked the responses “high blood pressure (that started during this pregnancy), pre-eclampsia or eclampsia” or “depression” were considered positive for these conditions.
Covariates
We selected potential confounders based on the 10% change-in-estimate criterion and clinical relevance (Citation30). Based on prior research, confounders that are associated with HDP and birth outcomes include maternal age, race/ethnicity, education level, cigarette use during last 3 months of pregnancy (yes or no), total household income in the 12 months before delivery, maternal pre-pregnancy body mass index (BMI), maternal weight gain, history of preterm birth (yes or no), infant sex (male or female), health insurance, prenatal care adequacy, and visit for depression or anxiety in the 12 months before pregnancy (yes or no) (Citation31,Citation32). Cigarette use, income, maternal BMI, health insurance and visit for depression or anxiety in the 12 months before pregnancy information were self-reported via the PRAMS questionnaire. Information on the remaining confounders was retrieved from the birth certificates.
For our analysis, maternal age was grouped to four categories: “less than 20 years,” “20 to 29 years,” “30–34 years” and “35 years and older.” Education level was categorized as “less than high school,” “high school graduate” and “college and above.” Race was recoded to five categories: “White,” “Black,” “Asian,” “other non-White” and “mixed race.” In accordance with the American College of Obstetricians and Gynecologists (ACOG) guidelines on weight gain during pregnancy, maternal weight gain was categorized as “meet recommendation,” “under recommendation” and “exceed recommendation” (Citation33). Prenatal care adequacy was assessed by the Kotelchuck Index from birth certificate data, and categorized as “inadequate (received <50% of expected visits),” “intermediate (50%−79%),” “adequate (80%−109%)” and “adequate plus (≥110%)” (Citation34). Total household income 12 months before delivery was recoded to “$20000 and less,” “$20000 to $ 40000” and “more than $40000.” Type of health insurance was categorized as “private/ACA (Affordable Care Act),” “Medicaid or other government insurance only,” “other,” “Medicaid and other” and “no insurance.” BMI was categorized as “underweight or normal weight (<25 kg/m2),” “overweight (25 – <30 kg/m2),” and “obese (≥30 kg/m2).”
Statistical analysis
We examined differences in sample characteristics by hypertension status during pregnancy using the Rao-Scott chi-square test for all (categorical) variables. Weighted percentages were used to describe the sample characteristics. We used modified Poisson regression model with robust error variance to examine the relationship between birth outcomes and HDP and DDP (Citation35). We chose Poisson regression because of the retrospective cohort study design to estimate the relative risk (RR). The modified Poisson model leads to narrower confidence intervals (CIs) compared with a simple Poisson model (Citation35). The following covariates were included in multivariable analysis to estimate the adjusted risk ratios (aRR): maternal age, race, education level, annual total household income, BMI, weight gain during pregnancy, previous preterm births, insurance type, diabetes during pregnancy, smoking in the last 3 months of pregnancy, infant sex, prenatal care adequacy, and healthcare visit for depression or anxiety 12 months before pregnancy. We performed interaction testing, including the estimation of both additive and multiplicative interaction effects, by adding an interaction term between HDP and DDP to a separate adjusted Poisson model for each of the four birth outcomes (Citation36). Using a format proposed by Knol and VanderWeele (Citation37), we reported aRRs and measures of interaction on both additive (relative excess risk due to interaction [RERI]) and multiplicative scales (ratio of RRs) with CIs and p-values. These estimates were generated using “interactionR” (Citation38), an open-source R package based on Knol and VanderWeele’s format, and the delta method from Hosmer and Lemeshow for the estimation of CIs of RERI (Citation39). About 24% of the original sample had at least one missing values. Removing these observations from the analysis could result in biased parameter estimates. Therefore, we used multiple imputation by chained equations (m = 20) to account for missing data (Citation40). A sensitivity analysis using complete-case analysis (n = 51,607) was also conducted to compare the estimated effects of HDP within the strata of DDP between complete-case analysis and the primary analysis with multiply-imputed data (n = 68,052). All statistical analyses were performed using R statistical software (version 4.0.2) and R Studio software (version 1.3.1073). The “survey” package was used to account for the weighting and survey design. α = 0.05 was considered statistically significant. The study protocol was reviewed by the Saint Louis University Institutional Review Board and was classified as exempt.
Results
The primary analysis using multiply-imputed data included 68,052 women who participated in PRAMS and had a singleton birth during 2016–2018. Of the sample, 11.7% had HDP, 11.8% had DDP, and 2.5% had both HDP and DDP (weighted percentages). Compared to women without HDP, those who had HDP are more likely to be younger than 20 years old or 35 years or older, of Black or mixed race, obese, had lower proportion of college and above level of education and lower annual total household income, had gestational weight gain exceeding recommendation, had a previous preterm birth, used Medicaid or other government insurance, had diabetes during pregnancy, smoked during pregnancy, had inadequate prenatal care, had DDP and sought care for depression or anxiety 12 months before pregnancy ().
Table 1. Characteristics of study sample by hypertension status (unweighted n = 68,052).
present the aRRs with CIs and p-values for the interaction between HDP and DDP on the risk of PTB and LBW, SGA, and LGA, respectively. Comparing women with HDP to those without HDP, the risk of PTB was 1.58 (95% CI 1.30, 1.92) in women with DDP and 2.58 (95% CI 2.34, 2.83) in women without DDP; the risk of LBW was 1.96 (95% CI 1.54, 2.50) in women with DDP and 4.03 (95% CI 3.59, 4.54) in women without DDP (); the risk of SGA was 1.27 (95% CI 1.02, 1.57) in women with DDP and 1.50 (95% CI 1.34, 1.67) in women without DDP; the risk of LGA was 0.92 (95% CI 0.70, 1.21) in women with DDP and 0.84 (95% CI 0.74, 0.96) in women without DDP ().
Table 2. Interaction between hypertension and depression during pregnancy on the risk of preterm birth (PTB) and low birth weight (LBW), PRAMS, 2016–2018 (n = 68,052).
Table 3. Interaction between hypertension and depression during pregnancy on the risk of small-for-gestational-age (SGA) and large-for-gestational-age (LGA), PRAMS, 2016–2018 (n = 68,052).
The RERIs for PTB, LBW, and SGA were below 0, which indicated negative interaction across strata of DDP on an additive scale. However, these measures were not statistically significant, including the positive one for LGA. The measure of interaction on multiplicative scale, the ratios of RRs, were 0.61 (95% CI 0.50, 0.76), 0.49 (95% CI 0.37, 0.63), 0.85 (95% CI 0.67, 1.08) and 1.10 (95% CI 0.81, 1.48) for PTB, LBW, SGA, and LGA, respectively. The former two were statistically significant (p < 0.01), indicating negative interaction effects on the multiplicative scale for PTB and LBW. This means that the estimated joint effect of HDP and DDP was smaller than the product of the estimated independent effect of HDP and DDP.
The estimated effects of HDP within the strata of DDP using complete-case data were consistent to the results of our primary analyses in terms of magnitude and direction, which did not change the interpretations of the findings ().
Table 4. Effect of hypertension during pregnancy within the strata of depression during pregnancy comparing complete-case data and multiply-imputed data.
Discussion
Our study found that women with HDP compared to those without, had increased risk of PTB, LBW, SGA but reduced risk of LGA, after controlling for confounders. The findings confirmed our hypothesis and were consistent with previous work (Citation3–5). We observed that DDP increased the risk of PTB, LBW, and SGA, though not statistically significant for SGA. Our findings were also in line with a previous study that reported no additive interaction effect of HDP and DDP on birth outcomes (Citation41). However, the adverse effects on birth outcomes among women with both conditions were lower than expected. We observed negative interaction of HDP and DDP on multiplicative scale for the risk of PTB and LBW. The joint effects were even lower than the independent effects of HDP on the risk of PTB, LBW, and SGA.
Although the causes of HDP and DDP among pregnant women are not well understood, some well-accepted pathophysiology for both HDP and DDP implicate shared physiological pathways. The abnormality of placentation that leads to reduced transfer of oxygen and nutrients to the developing fetus might trigger the maternal inflammatory response including endothelial dysfunction and increased blood pressure (Citation42). Recent studies have also shown that inflammation was associated with depressive symptoms and adverse birth outcomes (Citation43,Citation44). Thus, women who have both HDP and DDP are possibly at greater risk of adverse birth outcomes than those with only one condition.
Despite the increasing prevalence of depression among pregnancy-related hospitalizations, providers may address conditions such as hypertension or diabetes during pregnancy first. However, in many cases, they would ignore or delaying the treatment of depression when patients having both depression and other medical conditions (Citation45). The untreated DDP may lead to adverse birth outcomes and serious long-term effects on children (Citation23,Citation46). However, only few prior studies have been conducted that examined depression as an effect modifier in the association between HDP and adverse birth outcomes (Citation41). In a prospective cohort study in Canada of 2,763 women, Horsley et al (Citation25) observed that the joint impact of HDP and depression on shorter gestational age at birth increased as depressive symptoms became greater. Findings from Horsley et al were limited by low incidence of DDP and over-sampled white, married women with high socioeconomic status. Similarly, Mogos and colleagues (Citation41) reported that HDP and depression jointly increased risk of intrauterine growth restriction (IUGR), stillbirth, and PTB, although HDP was the main driving factor of the joint association over a 58-million nationwide inpatient sample. However, the cross-sectional nature of the study and failure to adjust for important confounders may have biased the study results.
The antagonistic joint effect of HDP and DDP on birth outcomes in our study was unexpected. One possible explanation is that the diagnosis of depression in women before or during pregnancy or the diagnosis of both HDP and DDP may lead to more medical screening, monitoring by the healthcare providers, and improved prenatal care that results in better pregnancy outcomes compared to healthy mothers (Citation45). In other words, the joint effect may not be attenuated by the condition (depression) itself, but the additional care or medication. Because specific information on medication and treatment received (e.g., type, procedure, duration) for depression is not available in our dataset, failing to control for these variables as confounders may bias the results toward the null value. In addition, failure to account for the severity of HDP in the study may have biased our results as well.
The strength of this study rests in its use of a large population-based sample of women across the U.S and the availability of hypertension and depression status during pregnancy as well as information on many potential confounders. The large sample size ensured sufficient statistical power to detect significant associations and increased precision in the risk estimates. Our study adds to the existing evidence by investigating the joint effect of HDP and DDP on major adverse birth outcomes, which is rarely examined in prior research. However, there are some limitations of the present study. Firstly, the presence of HDP and DDP were self-reported by simply checking options of a single question. It may underestimate the extent of HDP and DDP due to lack of information on severity and duration of the conditions. Additionally, the specific timing of onset of HDP and the diagnosis of DDP is not available in the PRAMS data. Previous studies have shown a stronger association between HDP and elevated risks of adverse birth outcomes in mid-to-late pregnancy (Citation6,Citation7,Citation9). A recent meta-analysis indicated that the risk of LBW was higher when depression was assessed in the first trimester compared to the risk measured in the second or third trimester (Citation47). It was also found that depressive symptoms were higher at the beginning and end of pregnancy (Citation48), and worsening symptoms during pregnancy may be associated with increased risks of adverse pregnancy outcomes (Citation49). Besides, the onset of HDP or DDP may increase the risk of the other, which may further complicate their effects on birth outcomes (Citation50). Therefore, given the effect of timing on varying risks of birth outcomes in existing evidence, future studies measuring HDP and DDP over time as pregnancy progresses are warranted. Similarly, potential information bias may be due to the self-report nature of the survey and birth certificate data. However, the misclassification of our data is likely non-differential which would bias the point estimates toward the null value. Future research using validated measurement for more objective data is warranted. Secondly, there may be an issue of residual confounding with our study findings due to lack of some behavioral risk factors during pregnancy (e.g., alcohol and substance use) and other relevant pregnancy history (e.g., history of LBW, stillbirth). In addition, the generalizability of this study should be interpreted with caution due to selection bias introduced by missing data. However, we addressed missingness with multiple imputation and our estimated effects of HDP within the strata of DDP from complete-case analysis were similar to those from the multiply-imputed analysis. Nevertheless, the proportion of African American women in our sample was still slightly greater than the national average for 2016–2018 (13.9% vs. 12%) (Citation51), which could possibly explain the high incidence of HDP in our sample because African Americans are more likely than Whites to suffer from hypertension. The final limitation is information bias resulting from misclassification of annual total household income, although we expected it would have a neglectable effect on our results.
This study confirmed findings from previous studies that women with HDP were at significantly higher risks of PTB, LBW, and SGA compared with women who do not have HDP. This highlighted the importance of early detection and management of HDP to reduce these adverse birth outcomes. We also found a significant independent effect of DDP on PTB and LBW but an unexpected antagonistic joint effect of HDP and DDP. Nonetheless, the joint effect on outcomes was greater than the independent effect of DDP on some adverse pregnancy outcomes. This highlighted the importance of depression screening among women with medical conditions for timely treatments and incentivized the development of tailored interventions in advance to maximize maternal and fetal outcomes. Future studies should examine the physiologic and pathological pathways through which depression and the concurrence of HDP and DDP affect pregnancy outcomes, as well as the persistent racial disparities in pregnancy outcomes.
The PRAMS working group representatives
Alabama – Tammie Yelldell, MPH; Alaska – Kathy Perham-Hester, MS, MPH; Arizona – Enid Quintana-Torres, MPH; Arkansas – Letitia de Graft-Johnson, DrPH, MHSA; Colorado – Ashley Juhl, MSPH; Connecticut – Jennifer Morin, MPH; Delaware – George Yocher, MS; Florida – Tara Hylton, MPH; Georgia – Florence A. Kanu, PhD, MPH; Hawaii – Matt Shim, PhD, MPH; Illinois – Julie Doetsch, MA; Indiana – Brittany Reynolds, MPH; Iowa – Jennifer Pham; Kentucky – Tracey D. Jewell, MPH; Louisiana – Rosaria Trichilo, MPH; Maine – Tom Patenaude, MPH; Maryland – Laurie Kettinger, MS; Massachusetts – Hafsatou Diop, MD, MPH; Michigan – Peterson Haak; Minnesota – Mira Grice Sheff, PhD, MS; Mississippi – Brenda Hughes, MPPA; Missouri – Venkata Garikapaty, PhD; Montana – Emily Healy, MS; Nebraska – Jessica Seberger; New Hampshire – David J. Laflamme, PhD, MPH; New Jersey – Sharon Smith Cooley, MPH; New Mexico – Sarah Schrock, MPH; New York State – Anne Radigan; New York City – Lauren Birnie, MPH; North Carolina – Kathleen Jones-Vessey, MS; North Dakota – Grace Njau, MPH; Oklahoma – Ayesha Lampkins, MPH, CHE; Oregon – Cate Wilcox, MPH; Pennsylvania – Sara Thuma, MPH; Puerto Rico – Wanda Hernandez, MPH; Rhode Island – Karine Tolentino Monteiro, MPH; South Carolina – Harley T. Davis, PhD, MPSH; South Dakota – Maggie Minett; Texas – Tanya Guthrie, PhD; Tennessee – Ransom Wyse, MPH, CPH; Utah – Nicole Stone, MPH; Vermont – Peggy Brozicevic; Virginia – Kenesha Smith, PhD, MSPH; Washington – Linda Lohdefinck; West Virginia – Melissa Baker, MA; Wisconsin – Fiona Weeks, MSPH; Wyoming – Lorie Chesnut, PhD; and the CDC PRAMS Team, Women”s Health and Fertility Branch, Division of Reproductive Health.
Acknowledgments
We would like to thank the PRAMS (Pregnancy Risk Assessment Monitoring System) Working Group for its role in conducting PRAMS surveillance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available via a data application to PRAMS at https://www.cdc.gov/prams/prams-data/researchers.htm.
Additional information
Funding
References
- Wilkerson RG, Ogunbodede AC. Hypertensive disorders of pregnancy. Emerg Med Clin North Am. 2019 May;37(2):301–10. doi: 10.1016/j.emc.2019.01.008
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011 Aug;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006
- Browne JL, Vissers KM, Antwi E, et al. Perinatal outcomes after hypertensive disorders in pregnancy in a low resource setting. Tropical Med Int Health. 2015 Dec;20(12):1778–1786.
- Lei F, Liu D, Shen Y, et al. Study on the influence of pregnancy-induced hypertension on neonatal birth weight. J Investig Med. 2018 Aug;66(6):1008–1014.
- Johnson KM, Zash R, Haviland MJ, et al. Hypertensive disease in pregnancy in Botswana: prevalence and impact on perinatal outcomes. Pregnancy Hypertens. 2016 Oct;6(4):418–422.
- Bakker R, Steegers EA, Hofman A, et al. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am J Epidemiol. 2011 Oct 1;174(7):797–806. doi: 10.1093/aje/kwr151
- Starling AP, Shapiro ALB, Sauder KA, et al. Blood pressure during pregnancy, neonatal size and altered body composition: the healthy start study. J Perinatol. 2017 May;37(5):502–506.
- Lu CQ, Lin J, Yuan L, et al. Pregnancy induced hypertension and outcomes in early and moderate preterm infants. Pregnancy Hypertens. 2018 Oct;14:68–71.
- Zhu B, Huang K, Bao W, et al. Dose-response relationship between maternal blood pressure in pregnancy and risk of adverse birth outcomes: ma’anshan birth cohort study. Pregnancy Hypertens. 2019 Jan;15:16–22.
- Dassah ET, Kusi-Mensah E, Morhe ESK, et al. Maternal and perinatal outcomes among women with hypertensive disorders in pregnancy in Kumasi, Ghana. PLoS One. 2019;14(10):e0223478. doi: 10.1371/journal.pone.0223478
- Davies EL, Bell JS, Bhattacharya S. Preeclampsia and preterm delivery: a population-based case-control study. Hypertens Pregnancy. 2016 Nov;35(4):510–519. doi: 10.1080/10641955.2016.1190846
- Bridwell M, Handzel E, Hynes M, et al. Hypertensive disorders in pregnancy and maternal and neonatal outcomes in Haiti: the importance of surveillance and data collection. Bmc Pregnancy Childbirth. 2019 Jun 20;19(1):208. doi: 10.1186/s12884-019-2361-0
- Xiong X, Fraser WD. Impact of pregnancy-induced hypertension on birthweight by gestational age. Paediatr Perinat Epidemiol. 2004 May;18(3):186–191. doi: 10.1111/j.1365-3016.2004.00553.x
- Powe CE, Ecker J, Rana S, et al. Preeclampsia and the risk of large-for-gestational-age infants. Am J Obstet Gynecol. 2011 May;204(5):425 e1–6.
- Giurgescu C, Engeland CG, Templin TN. Symptoms of depression predict negative birth outcomes in african american women: a pilot study. J Midwifery Women’s Health. 2015 Sep;60(5):570–577. doi: 10.1111/jmwh.12337
- Nutor JJ, Slaughter-Acey JC, Giurgescu C, et al. Symptoms of depression and preterm birth among black women. MCN The American J Maternal Child Nursing. 2018 Sep;43(5):252–258.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010 Oct;67(10):1012–1024.
- Staneva A, Bogossian F, Pritchard M, et al. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth. 2015 Sep;28(3):179–193.
- Accortt EE, Cheadle AC, Dunkel Schetter C. Prenatal depression and adverse birth outcomes: an updated systematic review. Maternal Child Health J. 2015 Jun;19(6):1306–1337. doi: 10.1007/s10995-014-1637-2
- Szegda K, Markenson G, Bertone-Johnson ER, et al. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern-Fetal Neonatal Med: The Official Journal Of The European Association Of Perinatal Medicine, The Federation Of Asia And Oceania Perinatal Societies, The International Society Of Perinatal Obstet. 2014 Jun;27(9):960–967.
- Ncube CN, Enquobahrie DA, Gavin AR. Racial differences in the association between maternal antenatal depression and preterm birth risk: a prospective cohort study. J Women’s Health. 2017 2002 Dec;26(12):1312–1318. doi: 10.1089/jwh.2016.6198.
- Abdelaal H, Mohamed MA, Aly H. Racial disparity, depression, and birth outcomes among pregnant teens. Maternal Child Health J. 2018 Oct;22(10):1400–1406. doi: 10.1007/s10995-018-2519-9
- Jarde A, Morais M, Kingston D, et al. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: a systematic review and meta-analysis. JAMA Psychiatry. 2016 Aug 1;73(8):826–837. doi: 10.1001/jamapsychiatry.2016.0934
- Mei-Dan E, Ray JG, Vigod SN. Perinatal outcomes among women with bipolar disorder: a population-based cohort study. Am J Obstet Gynecol. 2015 Mar;212(3):.e367.1–.e367.8. doi: 10.1016/j.ajog.2014.10.020
- Horsley KJ, Tomfohr-Madsen LM, Ditto B, et al. Hypertensive disorders of pregnancy and symptoms of depression and anxiety as related to gestational age at birth: findings from the all our families study. Psychosom Med. 2019 Jun;81(5):458–463.
- Hilmert CJ, Schetter CD, Dominguez TP, et al. Stress and blood pressure during pregnancy: racial differences and associations with birthweight. Psychosom Med. 2008 Jan;70(1):57–64.
- Shulman HB, D’Angelo DV, Harrison L, et al. The Pregnancy Risk Assessment Monitoring System (PRAMS): overview of design and methodology. Am J Public Health. 2018 Oct;108(10):1305–1313.
- WHO. Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended october 14, 1976. Acta Obstet Gynecol Scand. 1977;56(3):247–253. doi: 10.3109/00016347709162009
- Centers for Disease Control and Prevention. Pregnancy Risk Assessment Monitoring System (PRAMS) phase 8 core questionnaire 2016, [accessed 2020 Jul 3rd]. https://www.cdc.gov/prams/pdf/questionnaire/Phase-8-Core-Questions-508.pdf
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993 Dec 1;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813.
- Singh GK, Siahpush M, Liu L, et al. Racial/Ethnic, nativity, and sociodemographic disparities in maternal hypertension in the United States, 2014-2015. Int J Hypertens. 2018;2018:1–14. doi: 10.1155/2018/7897189
- Tanaka M, Jaamaa G, Kaiser M, et al. Racial disparity in hypertensive disorders of pregnancy in New York State: a 10-year longitudinal population-based study. Am J Public Health. 2007 Jan;97(1):163–170.
- American College of O. Gynecologists. ACOG committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013 Jan; 121 1:210–212.10.1097/01.AOG.0000425668.87506.4c.
- Kotelchuck M. An evaluation of the Kessner Adequacy of prenatal care index and a proposed adequacy of prenatal care utilization index. Am J Public Health. 1994 Sep;84(9):1414–1420. doi: 10.2105/AJPH.84.9.1414
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090.
- Brankovic M, Kardys I, Steyerberg EW, et al. Understanding of interaction (subgroup) analysis in clinical trials. Eur J Clin Invest. 2019 Aug;49(8):e13145.
- Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514–520. doi: 10.1093/ije/dyr218.
- Alli BY. InteractionR: an R package for full reporting of effect modification and interaction. Software Impacts. 2021;10:100147. doi: 10.1016/j.simpa.2021.100147
- Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012.
- van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Soft. 2011;45(3):1–67. 12/12. doi: 10.18637/jss.v045.i03
- Mogos MF, Jones LM, Robinson NS, et al. Prevalence, correlates, and outcomes of co-occurring depression and hypertensive disorders of pregnancy. J Women Health. 2019 Nov;28(11):1460–1467.
- Macdonald-Wallis C, Tilling K, Fraser A, et al. Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: findings from a prospective cohort. Hypertension. 2014 Jul;64(1):36–44.
- Ilekis JV, Tsilou E, Fisher S, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an executive workshop summary of the Eunice Kennedy shriver national institute of child health and human development. Am J Obstet Gynecol. 2016 Jul;215(1 Suppl):S1–S46.
- Osborne LM, Monk C. Perinatal depression–the fourth inflammatory morbidity of pregnancy?: theory and literature review. Psychoneuroendocrinology. 2013 Oct;38(10):1929–1952. doi: 10.1016/j.psyneuen.2013.03.019
- Kravitz RL, Ford DE. Introduction: chronic medical conditions and depression—the view from primary care. Am j med. 2008 Nov;121(Suppl 11):S1–7. doi: 10.1016/j.amjmed.2008.09.007
- Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. 2017 Feb 7;342:154–166.
- Ghimire U, Papabathini SS, Kawuki J, et al. Depression during pregnancy and the risk of low birth weight, preterm birth and intrauterine growth restriction- an updated meta-analysis. Early Hum Dev. 2021 Jan;152:105243.
- Amiel Castro RT, Pinard Anderman C, Glover V, et al. Associated symptoms of depression: patterns of change during pregnancy. Arch Womens Ment Health. 2017 Feb;20(1):123–128.
- Miller ES, Saade GR, Simhan HN, et al. Trajectories of antenatal depression and adverse pregnancy outcomes. Am J Obstet Gynecol. 2022 Jan;226(1):.e108.1–.e108.9.
- Cripe SM, Frederick IO, Qiu C, et al. Risk of preterm delivery and hypertensive disorders of pregnancy in relation to maternal co-morbid mood and migraine disorders during pregnancy. Paediatr Perinat Epidemiol. 2011 Mar;25(2):116–123.
- KFF’s State Health Facts. Data source: kaiser family foundation estimates based on the census Bureau’s American community survey -. population distribution by race/Ethnicity, 2016-2018. [accessed 2020 Jul 3rd]. https://www.kff.org/other/state-indicator/distribution-by-raceethnicity/?activeTab=graph¤tTimeframe=0&startTimeframe=2&selectedDistributions=black&selectedRows=%7B%22wrapups%22:%7B%22united-states%22:%7B%7D%7D%7D&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D