ABSTRACT
Objective
To assess changes in expression of renal epithelial sodium channel (ENaC) and NEDD4L, a ubiquitin ligase, in urinary extracellular vesicles (UEV) of pre-eclamptic women compared to normal pregnant controls.
Methods
Urine was collected from pre-eclamptic women (PE, n = 20) or during normal pregnancy (NP, n = 20). UEV were separated by differential ultracentrifugation. NEDD4L, α-ENaC and γ-ENaC were identified by immunoblotting.
Results
There was no difference in the expression of NEDD4L (p = 0.17) and α-ENaC (p = 0.10). PE subjects showed increased expression of γ-ENaC by 6.9-fold compared to NP (p < 0.0001).
Conclusion
ENaC expression is upregulated in UEV of pre-eclamptic subjects but was not associated with changes in NEDD4L.
Introduction
Despite suppression of the renin-angiotensin-aldosterone axis, patients with pre-eclampsia display increased urine sodium retention, indicating a state of altered renal salt and water homeostasis (Citation1). The pathophysiology of pre-eclampsia is complex and the syndrome is characterized by hypertension occurring after 20 weeks gestation, in combination with organ involvement and/or fetal growth restriction (Citation2). The epithelial sodium channel (ENaC) is a key transporter governing sodium reabsorption in the distal convoluted tubule and collecting duct of the nephron (Citation3). Recent understanding of its regulation by NEDD4–2 (human NEDD4L), a ubiquitin ligase, may provide greater understanding into the pathogenesis of pre-eclampsia.
NEDD4–2 is a protein of the neuronal precursor cell-expressed developmentally down-regulated 4 (NEDD4) family, which are ubiquitin ligases (Citation4). They share a similar molecular structure with an N-terminal C2 domain, several WW domains, and a C-terminal HECT-type ubiquitin ligase domain (Citation5). Ubiquitin ligases are essential for the process of ubiquination as they catalyze the transfer of ubiquitin to a substrate protein, which marks it for degradation or recognition by specific signaling molecules to alter trafficking (Citation5). One of the best characterized substrates of NEDD4–2 is ENaC.
The ENaC transporter is composed of three subunits - α, β, γ (Citation6). Each subunit consists of intracellular N and C termini, two transmembrane domains, and a large extracellular loop. The C terminal tails contain PY motifs which serve as the binding sites for the WW domains of NEDD4–2 (Citation4). This interaction results in the transfer of ubiquitin and subsequent internalization and degradation of ENaC (Citation5). The importance of the NEDD4–2/ENaC interaction is evident in Liddle’s syndrome, where deletions or substitutions in the PY motif of β- or γ-ENaC leads to an inability of NEDD4–2/NEDD4L to bind (Citation4). Failure to internalize ENaC from the cell surface despite volume expansion results in the clinical presentation of hypertension, hypokalemia, and metabolic alkalosis (Citation4). In Nedd4–2 knockout mice, a similar phenotype of salt-sensitive hypertension has been observed (Citation7). The animals display increased retention of mature and active forms of all three subunits of ENaC at the apical membrane in the tubular epithelia (Citation7). Furthermore, Nedd4–2 deficient mice develop progressive kidney disease, which is improved by administration of the ENaC antagonist, amiloride, and exacerbated by high dietary sodium (Citation8,Citation9).
Aside from NEDD4–2, there are multiple other mechanisms that regulate ENaC activity (Citation6). Proteolytic cleavage by proteases such as furin, prostatin, and plasmin remove inhibitory domains in the extracellular loop of ENaC leading to activation of the channel (Citation6). Buhl et al. demonstrated significantly elevated plasmin in the urine of pre-eclamptic subjects (Citation10). NEDD4–2 itself is regulated by other proteins and hormones, such as aldosterone and insulin (Citation5). In response to aldosterone, the serum and glucocorticoid-regulated kinase 1 (Sgk1) phosphorylates NEDD4–2, resulting in loss of its function and, in turn, higher ENaC membrane retention (Citation6).
Previous studies by Nielsen et al. and Hu et al. showed increased expression of α-ENaC and γ-ENaC in urinary extracellular vesicles (UEV) of pre-eclamptic subjects compared to normotensive pregnant women and normal controls (Citation11,Citation12). These studies suggest that renal sodium transporters play a role in avid sodium retention and hypertension in pre-eclampsia, and that ENaC is likely to be of particular importance.
UEV are small membrane-enclosed particles released into the urine by cells lining the kidney and urinary tract (Citation13,Citation14). The extracellular vesicle population comprises a mixture of microvesicles, exosomes, and apoptotic bodies, which differ in their origin and size (Citation14). The components include cell surface markers, cytoplasmic proteins, and nuclear material, which are enclosed in a lipid bilayer and, hence, relatively stable to degradation (Citation15). Due to their small size, UEV are most commonly separated from whole urine by differential centrifugation which involves a sequence of high-speed ultracentrifugation steps (Citation13). Proteins can then be identified by immunoblotting, reflecting the protein expression profile of the cells of origin (Citation15,Citation16).
Given the importance of NEDD4–2/NEDD4L in regulating the degradation of ENaC, this study aimed to compare the expression of NEDD4L and ENaC in UEV of pre-eclamptic subjects and normotensive pregnant controls.
Materials and methods
This cross-sectional study was approved by the Mercy Health Human Research Ethics Committee (R14–28) and written informed consent was obtained. Participants with pre-eclampsia (PE) as per diagnostic criteria from the Society of Obstetric Medicine of Australia and New Zealand were recruited (n = 20) (Citation17). The control group consisted of normotensive pregnant (NP) women (n = 20). Other inclusion criteria included age greater than 18 years, second or third trimester and no history of cardiac disease, renal disease, or diabetes. Patients were recruited from the Mercy Hospital for Women between 1 May 2015 to 30 July 2021.
Sample collection
Fifty milliliters of urine were collected from each participant in a sterile container and immediately placed on ice. The sample was centrifuged at 1,600 × g at 4°C for ten minutes to remove cellular debris (Megafuge 1.0 R, Heraeus). One milliliter of urine was reserved and stored at −80°C for urine biochemistry. Protease inhibitor 400 µg/mL (Sigma-Aldrich protease inhibitor cocktail P8340, Missouri, USA) was added to the remaining supernatant (125 µL/10 mL) and the sample was frozen at −80°C for processing at a later date.
Separation of UEV
Separation of UEV was by differential ultracentrifugation, as has been described (Citation12,Citation13,Citation18). In brief, frozen urine (10 mL) was vortexed vigorously until thawed, then aliquoted into three 3 mL Beckman polycarbonate tubes and centrifuged at 17,000 × g at 4°C for 15 minutes (Optima TLX 120CE ultracentrifuge, Beckman Coulter, Inc). The supernatant was placed into three new polycarbonate tubes and weight balanced again. The pellet was discarded. Samples were ultracentrifuged at 200,000 × g at 4°C for 60 minutes to pellet UEV. The supernatant was discarded and the pellet resuspended in 1 mL of phosphate buffered saline supplemented with protease inhibitor at a concentration of 125 µL/10 mL. The tubes were vortexed for 30 seconds and the samples were pooled into one new polycarbonate tube with a resulting volume of 3 mL. This sample was ultracentrifuged again at 200,000 × g at 4°C for 60 minutes. The pellet was resuspended in 40 µL PBS/PI and vortexed for 30 seconds. Subsequently, 50 µL was placed into an Eppendorf tube, and 14 µL SDS sample buffer (1:5 volume) and 6 µL 400 mg/mL dithiothreitol was added. The final sample was vortexed for 30 seconds and then stored at −80°C.
Immunobotting
Samples were removed from −80°C storage, heated for five minutes at 90°C and vortexed for 30 seconds. Aliquots of 20 µL were loaded into a 10-well polyacrylamide gel and placed into a BioRad® Mini-Protean 3 Cell running tank with SDS running buffer. The first well of each gel was loaded with 20 µL of Precision Plus pre-stained protein standard (molecular weight 10-250kDa) to provide a reference for protein size. The BioRad cell was connected to a power supply at a constant 140 V for 1 hour and 15 minutes. The gel was placed on a polyvinylidene difluoride transfer membrane (PVDF) and transferred using a Transblot Turbo transfer system for 30 minutes at 10 V and 1 mA. After transfer was complete, the membrane was placed in blocking solution consisting of tris buffered saline with 0.05% tween-20 (TBST) and 10% bovine serum albumin (BSA) for at least one hour on a rotatory platform.
The membrane was incubated in an antigen-specific primary antibody (5% BSA in 10 mL TBST with 0.1% sodium azide) for one hour on a rotatory platform or overnight at 4°C on a roller mixer. The primary antibodies used included NEDD4–2/NEDD4L as previously described (Citation19), α-ENaC, γ-ENaC, as well as vesicle markers CD9 and TSG101 (). After washing, the membrane was incubated with a secondary swine anti-rabbit antibody conjugated to horseradish peroxidase (Dako, Glostrup, Denmark) for 45 minutes at room temperature on a rotatory platform. Membranes were incubated with chemiluminescent solution for two minutes, exposed to film, and developed through an automated developer (AGFA).
Table 1. Primary antibodies.
Films were scanned using Adobe Photoshop CS3 (version 10.0.1.0) and densitometry was analyzed using ImageJ software (version 1.4.3.67). Although multiple bands were present, only the 30kDa band of α-ENaC and 70kDa band of γ-ENaC were quantified as these represent the mature forms, which have been activated by proteolytic cleavage forms (Citation8). If no band was detectable, densitometry was recorded as zero. Spot urine creatinine was analyzed in the NATA accredited laboratory of Austin Health using a Beckman Coulter AU5800 auto analyzer. Densitometry data were expressed as arbitrary units relative to urine creatinine, which was used as a normalization marker for UEV (Citation20).
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 9.2.0. Unpaired t-tests were used to compare demographic data, and categorical variables were analyzed using the Fisher’s exact test. The Mann Whitney U test was used to compare protein expression between the two groups (NP vs PE), in view of the non-parametric distribution. The percentage of samples that had bands present or absent was analyzed using contingency tables and Fisher’s exact test. Confidence intervals were calculated using the modified Wald method. Linear regressions between NEDD4L with α- and γ-ENaC was performed to determine any correlations. A p value < 0.05 was considered significant.
Results
Subject characteristics are shown in . Samples which did not have vesicle marker expression were excluded from analysis, resulting in 17 and 19 participants in the NP and PE groups, respectively. The average age in the two groups were similar. PE patients were of earlier gestation at 30.3 weeks compared to 37.1 weeks in the NP group (p < 0.001) and had a significantly elevated blood pressure (p < 0.0001). The median urine protein/creatinine ratio was 135 mg/mmol in the PE group, and there was no difference in the spot urine creatinine between the two groups (p = 0.89). There was also no difference in the body mass index (= 0.06). Forty-seven percent of babies from the PE group had a birthweight lower than 10th percentile adjusted for gestational age, compared to the NP group where all birthweights were either normal or large for gestational age (p < 0.01). Regarding serum biochemistry, PE subjects had a significantly higher serum potassium (p = 0.03) but no difference in serum bicarbonate (p = 0.13). The PE subjects also had a significant lower serum sodium (p = 0.03). There was no statistical difference in serum creatinine (p = 0.10).
Table 2. Characteristics of subjects in the normotensive pregnant and pre-eclamptic groups.
A representative immunoblot summarizing the main observations of four NP and four PE subjects with antibodies against NEDD4L, α-ENaC, and γ-ENaC, as well as vesicle markers TSG101 and CD9 are shown in . Densitometry analyses showed no difference between NP and PE in the expression of NEDD4L (110 and 130kDa bands) (p = 0.17) and α-ENaC (p = 0.10) (). In the PE group, there was significantly increased expression of mature-form γ-ENaC by 6.9-fold compared to NP (p < 0.0001) (). When analyzing whether the mature cleaved forms of α-ENaC or γ-ENaC was present or absent, there was a significantly increased percentage of expression of both subunits in PE (). The α-ENaC subunit was expressed in 47% of PE patients (95% CI 27.4, 68.2) and 24% of NP patients (95% CI 9.2, 47.9). The γ-ENaC subunit was expressed in 95% of PE patients (95% CI 73.2, 100.7) and 17% of NP patients (95% CI 5.6, 42.0). Full images of uncropped Western Blots are provided in Supplementary Figure S1.
Figure 1. Expression of antibodies directed against NEDD4L, α-ENaC and γ-ENaC on Western blot in normotensive pregnant controls (NP, n = 17) and pre-eclamptic patients (PE, n = 19) normalized to spot urine creatinine (mmol/L). Data are presented as scatter plots, with each patient’s densitometry value represented as a dot and the horizontal line is the median of the sample. (a) A representative Western blot of four NP patients and four PE patients with antibodies against NEDD4L, α-ENaC, and γ-ENaC, as well as vesicle markers TSG101 and CD9. (b) Densitometry analysis of NEDD4L (110 and 130kDa bands) showed no difference between the two groups (p = 0.17). (c) The 30kDa band of α-ENaC was quantified. Densitometry analysis did not show a significantly increased expression of α-ENaC in PE group compared to NP (p = 0.10). (d) For γ-ENaC, the 70kDa band was quantified. Densitometry analysis showed a 6.9-fold increased expression of γ-ENaC in PE group compared to NP (p < 0.0001). (e) Percentage of α-ENaC expression in NP and PE groups. (f) Percentage of γ-ENaC expression in NP and PE groups.
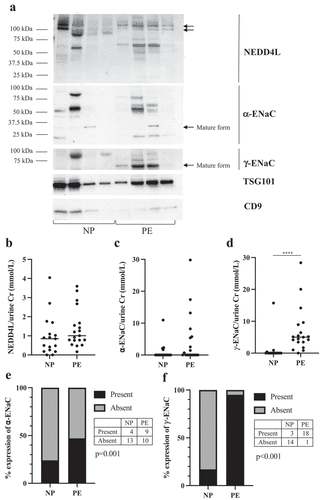
Interestingly, in the PE subjects, we observed multiple lower molecular weight bands between 25 and 75kDa of NEDD4L as shown in , which were significantly different compared to the NP cohort (p < 0.001); however, the significance of these lower bands is unclear.
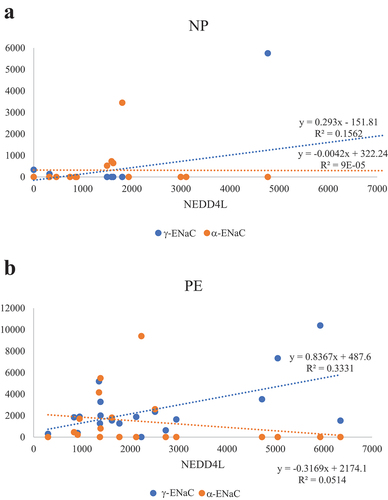
Regression analysis in the PE group of NEDD4L and detectable α- and γ-ENaC showed a weak positive correlation between NEDD4L and γ-ENaC which was statistically significant (R2 = 0.33, p = 0.01) (). In the NP group, there were no significant correlations between NEDD4L and α-ENaC (p = 0.97) or γ-ENaC (p = 0.12) ().
Figure 2. Regression analyses of NEDD4L with detectable α-ENaC and γ-ENaC in normotensive pregnant controls (NP) and pre-eclamptic patients (PE). (a) In the normotensive pregnant group, there were no significant correlations between NEDD4L and α-ENaC (p = 0.97), as well as NEDD4L and γ-ENaC (p = 0.12). (b) In the pre-eclampsia group, there was a significant correlation between Nedd4-2 and γ-ENaC (p = 0.01) but not with NEDD4L and α-ENaC (p = 0.35). Patients without detectable ENaC were not included in the regression analysis.
Discussion
This study confirmed that there is increased ENaC expression detectable in UEV in pre-eclampsia, consistent with previous reports (Citation11,Citation12). Firstly, Nielsen et al. demonstrated increased expression of the cleaved form of α-ENaC in urinary exosomes from pre-eclamptic subjects and pregnant controls, compared to a non-pregnant control group (Citation11). For γ-ENaC, the full-length form (90-100kDa) was detected in human renal cortical tissue, but only bands at 75, 60, and 37kDa were detected in urinary exosomes, indicating post-renal cleavage of γ-ENaC by proteases such as by plasmin (Citation11). Cleavage is associated with increased activity of ENaC subunits.
In an earlier separate cohort of subjects, our group compared renal sodium transporters expressed in UEV of pre-eclamptic and normotensive pregnant women as well as normal controls (Citation12). There was increased expression of α-ENaC, γ-ENaC, and total and phosphorylated Na-K-2Cl co-transporter 2 (NKCC2) in pre-eclampsia, but no difference in the expression of the thiazide-sensitive Na-Cl co-transporter (NCC). Hu et al. also detected and quantified multiple bands when probing for α-ENaC and γ-ENaC, which is different to the present study where only the mature forms were quantified.
In other clinical situations, ENaC over-expression manifests as hypertension, hypokalaemia, and metabolic alkalosis (Citation6,Citation9). Hypokalaemia and metabolic alkalosis are not typically described in pre-eclampsia. Upon comparing serum biochemistry in this study, we found higher serum potassium in the PE group and no difference in serum bicarbonate. The clinical manifestations in pre-eclampsia is complex and hyperkalemia could reflect evolving acute kidney injury. Serum sodium was lower in the PE group, which is reported to be a severe manifestation of PE (Citation21).
We hypothesized that increased ENaC expression in pre-eclampsia might be associated with reduced expression of NEDD4L; however, this was not found to be the case, as analyzed in UEVs.
There are multiple possible explanations for our observation that NEDD4L was not lower in pre-eclampsia. A urine sample contains a summation of all UEV released by cells lining the kidney and urinary tract (Citation14). While ENaC expression is restricted to the distal nephron, NEDD4L expression is found in the distal nephron, but also in the glomerulus, blood vessels and other cells (Citation9). In addition to ENaC, NEDD4–2 has been shown in vitro to ubiquitinate other substrates including signaling molecules, chloride channels, potassium channels, and transporters (Citation5). Therefore, a possible explanation for the lack of the predicted relationship between ENaC and NEDD4L expression in UEV could be due to NEDD4L expression in other kidney cells where it may be regulating other substrates. A second possibility is that the methodology was limited to full-length NEDD4L expression, without assessing its phosphorylation state, which can also affect activity (Citation5,Citation22). Indeed, phosphorylation of NEDD4–2 by signaling kinases, Sgk1 and Akt, has been shown to prevent the interaction of NEDD4–2 with ENaC (Citation5). Given NEDD4–2 is under the regulation of aldosterone, which is suppressed in pre-eclampsia, this may explain why NEDD4L expression remained persistent rather than down-regulated (Citation1,Citation5). Additional mechanisms of NEDD4–2 regulation, such as auto-ubiquination, oxidative stress, and 14-3-3 protein binding, may inhibit or sequester this protein, limiting its effect on ENaC (Citation5,Citation22). Furthermore, the role of proteolytic activation of ENaC, such as by plasmin, may be particularly important in pre-eclampsia and hence, the effect of NEDD4L is less clear (Citation10,Citation11).
The quantification of mature ENaC bands requires discussion. All three subunits of ENaC are post-translationally modified into mature forms. The α- and γ-subunits require proteolytic cleavage, whilst the β-subunit undergoes changes in glycosylation (Citation9,Citation23). Regarding α-ENaC, the primary antibody used in this study was directed against the N-terminal amino acids 46–68. Furin cleaves α-ENaC at two sites, resulting in forms of 75-85kDa (full-length), 60, and 30kDa (Citation23,Citation24). Regarding γ-ENaC, the primary antibody used in this study was against C-terminal amino acids 629–650. This subunit is cleaved by furin at a single site and by other proteases including prostasin, channel-activating protease 2, elastase, and plasmin (Citation24). This results in protein bands at 90kDa (full-length) and 70kDa (Citation23,Citation25). In a study of sodium depletion in mice, the presence of 30kDa α-ENaC and 70kDa γ-ENaC was increased, indicating that these were the mature activated forms (Citation23). By contrast, the 90kDa γ-ENaC decreased (Citation23). Furthermore, Zhou et al. showed that that NEDD4–2 regulated the mature complexes of ENaC located at the cell surface membrane but did not regulate the immature full-length form (Citation26). Hence, similar to studies by Henshall et al. and Manning et al., the 30kDa bands of α-ENaC and 70kDa bands of γ-ENaC were selectively quantified in the present study (Citation8,Citation9).
In urinary exosomes, Nielsen et al. found the 50kDa band of α-ENaC was significantly elevated in pregnancy, as well as 75, 60, and 37kDa forms of γ-ENaC. The primary antibodies used in that study were in-house monoclonal mouse anti-human antibodies directed at C terminal amino acids 436–445 of α-ENaC and N terminal amino acids 129–160 of γ-ENaC. This explains why the detected bands of each subunit were different in the present study to that found by Nielsen et al.
A limitation of this study is the small sample size. There were four urine samples which yielded negative vesicles markers, despite repeating the separation process twice. These samples had relatively low urine creatinine indicating these were more dilute and were likely to have a lower concentration of UEV. Additionally, Blijdorp et al. detected more Tamm-Horsfall protein in dilute urine, which generated more Tamm-Horsfall protein oligomers and interfered with the detection of UEV (Citation20). In the other samples, the vesicle markers TSG101 and CD9 were reliably detected, which supported the presence of UEV after the separation process. Further investigation such as with a larger study population would help identify any relationship between NEDD4L and α-ENaC. This would also enable comparisons between different clinical presentations of pre-eclampsia, such as early-onset versus term pre-eclampsia, and mild versus severe disease. The current study excluded participants with baseline renal disease or diabetes; however, analysis of NEDD4L and ENaC in these subgroups would provide interesting insights. Although there was no statistical difference in body mass index between the groups, future studies could examine whether pre-pregnancy obesity impacts ENaC and NEDD4L expression in pre-eclampsia.
Alterations in NEDD4L during normal pregnancy have not previously been described. Another limitation of this study is that we did not include a non-pregnant control group. Therefore, a future study evaluating the expression of NEDD4L in a non-pregnant normal control group would enable assessment of changes in NEDD4L in normal pregnancy. It was previously assumed that the UEV proteome reflected changes occurring in the kidney. Recently, Wu et al. demonstrated positive correlations between proteins expressed in UEV with paired kidney tissue (Citation27). Changes in protein expression after a normal or high potassium diet in an animal model were similarly reflected in UEV. In particular, transmembrane proteins and exosome markers had high correlation. By contrast, cytosolic proteins including ubiquitin E3 ligases had negative correlations between abundance in UEV and in kidney tissue. This confirms that UEV likely provides a reliable quantification of ENaC which is a transmembrane protein, but may be less reliable to assess NEDD4L, potentially contributing to the findings in this study.
In conclusion, ENaC expression is upregulated in UEV of pre-eclamptic subjects but this is not associated with changes in UEV full-length NEDD4L expression. Current understanding of the critical role of NEDD4–2/NEDD4L and regulation of ENaC have been derived from gene knockout studies in mice, genetic studies in Liddle’s syndrome, and single nucleotide polymorphisms in the Nedd4l gene on chromosome 18 associated with hypertension in humans (Citation28). To our knowledge, this is the first study investigating the NEDD4L/ENaC relationship in pre-eclampsia and there may be unique differences in its regulation in this condition and pregnancy.
Authors’ contributions
PL: separation of urinary extracellular vesicles, immunoblot analysis and densitometry, statistical analysis, preparation of figures and tables, initial draft of manuscript, revisions to manuscript, approval of final draft of manuscript. MK: contribution to study design, separation of urinary extracellular vesicles, Western blot analysis and densitometry, statistical analysis, revisions to manuscript, approval of final draft of manuscript. SWC: identification of potential participants, revisions to manuscript, approval of final draft of manuscript. NC: contribution to study design, revisions to manuscript, approval of final draft of manuscript. ML: revisions to manuscript, approval of final draft of manuscript. KP: identification of potential participants, revisions to manuscript, approval of final draft of manuscript. AA: identification and recruitment of participants, revisions to manuscript, approval of final draft of manuscript. JM: contribution to study design, immunoblot analysis and interpretation, revisions to manuscript, approval of final draft of manuscript. PM: contribution to study design, statistical analysis, preparation of figures and tables, revisions to manuscript, approval of final draft of manuscript. DP: initiation of study design, overall supervision of the project, statistical analysis, revisions to manuscript, primary responsibility for final draft of manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download MS Power Point (12.4 MB)Acknowledgments
This work was supported by a Research Training Program Scholarship from the University of Melbourne.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2232029.
Additional information
Funding
References
- Brown MA, Wang J, Whitworth JA. The renin-angiotensin-aldosterone system in pre-eclampsia. Clin Exp Hypertens. 1997 Jul;19(5–6):713–9. doi: 10.3109/10641969709083181
- Lowe SA, Bowyer L, Lust K, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015 Oct;55(5):e1–29.
- Busst CJ. Blood pressure regulation via the epithelial sodium channel: from gene to kidney and beyond. Clin Exp Pharmacol Physiol. 2013 Aug;40(8):495–503. doi: 10.1111/1440-1681.12124
- Rotin D, Staub O. Nedd4-2 and the regulation of epithelial sodium transport. Front Physiol. 2012;3:212. doi: 10.3389/fphys.2012.00212
- Manning JA, Kumar S. Physiological functions of Nedd4-2: lessons from knockout mouse models. Trends Biochem Sci. 2018 Aug;43(8):635–647. doi: 10.1016/j.tibs.2018.06.004
- Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008 Oct;19(10):1845–1854. doi: 10.1681/ASN.2008020225
- Shi PP, Cao XR, Sweezer EM, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol. 2008 Aug;295(2):F462–70.
- Manning JA, Shah SS, Henshall TL, et al. Dietary sodium modulates nephropathy in Nedd4-2-deficient mice. Cell Death Differ. 2020 Jun;27(6):1832–1843.
- Henshall TL, Manning JA, Alfassy OS, et al. Deletion of Nedd4-2 results in progressive kidney disease in mice. Cell Death Differ. 2017 Dec;24(12):2150–2160.
- Buhl KB, Friis UG, Svenningsen P, et al. Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension. 2012 Nov;60(5):1346–1351.
- Nielsen MR, Frederiksen-Moller B, Zachar R, et al. Urine exosomes from healthy and hypertensive pregnancies display elevated level of alpha-subunit and cleaved alpha- and gamma-subunits of the epithelial sodium channel-ENaC. Pflugers Arch. 2017 Sep;469(9):1107–1119.
- Hu CC, Katerelos M, Choy SW, et al. Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles. PLoS One. 2018;13(9):e0204514. doi: 10.1371/journal.pone.0204514
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004 Sep 7;101(36):13368–13373. doi: 10.1073/pnas.0403453101
- Erdbrugger U, Blijdorp CJ, Bijnsdorp IV, et al. Urinary extracellular vesicles: a position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J Extracell Vesicles. 2021 May;10(7):e12093.
- El Fekih R, Hurley J, Tadigotla V, et al. Discovery and validation of a urinary exosome mRNA signature for the diagnosis of human kidney transplant rejection. J Am Soc Nephrol. 2021 Mar 3;32(4):994–1004. doi: 10.1681/ASN.2020060850
- Merchant ML, Rood IM, Deegens JKJ, et al. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. 2017 Dec;13(12):731–749.
- Lowe SA, Bowyer L, Lust K, et al. The SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015 Feb;55(1):11–16.
- Ellis R, Katerelos M, Choy SW, et al. Increased expression and phosphorylation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoforms in urinary exosomes in pre-eclampsia. J Transl Med. 2019 Feb 28;17(1):60. doi: 10.1186/s12967-019-1806-6
- Konstas AA, Shearwin-Whyatt LM, Fotia AB, et al. Regulation of the epithelial sodium channel by N4WBP5A, a novel Nedd4/Nedd4-2-interacting protein. J Biol Chem. 2002 Aug 16;277(33):29406–29416. doi: 10.1074/jbc.M203018200
- Blijdorp CJ, Tutakhel OAZ, Hartjes TA, et al. Comparing approaches to normalize, quantify, and characterize urinary extracellular vesicles. J Am Soc Nephrol. 2021 May 3;32(5):1210–1226. doi: 10.1681/ASN.2020081142
- Powel JE, Rosenthal E, Roman A, et al. Preeclampsia and low sodium (PALS): a case and systematic review. Eur J Obstet Gynecol Reprod Biol. 2020;249:14–20. doi: 10.1016/j.ejogrb.2020.03.052.
- Pohl P, Joshi R, Petrvalska O, et al. 14-3-3-protein regulates Nedd4-2 by modulating interactions between HECT and WW domains. Commun Biol. 2021 Jul 22;4(1):899. doi: 10.1038/s42003-021-02419-0
- Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006 Sep;291(3):F683–93. doi: 10.1152/ajprenal.00422.2005
- Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009 Jul 31;284(31):20447–20451. doi: 10.1074/jbc.R800083200
- Hughey RP, Mueller GM, Bruns JB, et al. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003 Sep 26;278(39):37073–37082. doi: 10.1074/jbc.M307003200
- Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem. 2007 Jul 13;282(28):20207–20212. doi: 10.1074/jbc.M611329200
- Wu Q, Poulsen SB, Murali SK, et al. Large-scale proteomic assessment of urinary extracellular vesicles highlights their reliability in reflecting protein changes in the kidney. J Am Soc Nephrol. 2021 Sep;32(9):2195–2209.
- Goel P, Manning JA, Kumar S. NEDD4-2 (NEDD4L): the ubiquitin ligase for multiple membrane proteins. Gene. 2015 Feb 15;557(1):1–10. doi: 10.1016/j.gene.2014.11.051
- Joseph FA, Hyett JA, Schluter PJ, et al. New Australian birthweight centiles. Med J Aust. 2020 Jul;213(2):79–85.

