ABSTRACT
Objective
To study associations between the first-trimester maternal determinants of renin-angiotensin-aldosterone system (RAAS) activation and telomere length (TL) in pregnancies conceived natural and after IVF/ICSI.
Methods
In 145 pregnancies of the Rotterdam Periconception cohort renin, prorenin and aldosterone concentrations were measured in maternal blood at 9 weeks gestational age (GA). TL was measured by qPCR at 20 weeks GA.
Results
A significantly negative correlation was found between renin and TL, which was attenuated for prorenin but not observed for aldosterone. Maternal TL was significantly shorter in pregnancies conceived after in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) compared to natural pregnancies.
Conclusion
The negative association between first-trimester maternal renin and maternal TL, and the shorter maternal TL in women after IVF/ICSI treatment compared to natural pregnancies, substantiates the role of excessive RAAS activation.
Introduction
Pregnancy is characterized by systemic hemodynamic and cardiovascular adaptations, involving renal sodium retention and plasma volume expansion (PVE). Activation of the maternal renin-angiotensin-aldosterone system (RAAS) plays an important role in the periconceptional adaptation (Citation1,Citation2). After ovulation, the systematic hemodynamic adaptations that occur are less but comparable to the pregnancy situation. At 6 weeks after conception, a significant PVE occurs and evidence demonstrates that (Citation3) inadequate systematic hemodynamic adaptations are associated with adverse pregnancy outcomes, such as pre-eclampsia (PE) and fetal growth restriction (FGR) (Citation4–8).
The RAAS is a signaling cascade that plays an important role in electrolyte balance and regulating blood pressure. Increased vasodilation and lower blood pressure result in increased release of renal renin and its precursor prorenin, followed by increased generation of angiotensin II (Ang II) and aldosterone. Ang II is the major biologically active component of the RAAS and an important blood pressure regulator with effects on fluid and electrolyte balance through aldosterone increase (Citation9) (Citation10). Furthermore, it has been described, That the ovaries are an extra source of prorenin (Citation9,Citation11).
Excessive oxidative stress is due to an imbalance between reactive oxygen species (ROS) and antioxidants DNA, lipids, and proteins are structures that are most sensitive to excessive oxidative stressors. Numerous studies have shown that RAAS activation increases ROS production. Ang II, through several mechanisms, contributes to the increased formation of ROS (Citation12,Citation13). Activation of RAAS also stimulates the release of inflammatory cytokines, resulting in an increased inflammatory state (Citation14). Moreover, ovarian stimulation treatment can result in significantly higher RAAS activation during pregnancy and as such contributes to excessive oxidative stress and inflammation (Citation11).
Accelerated attrition of telomeres has been described as a consequence of (chronic) exposure to excessive oxidative DNA damage and inflammation (Citation15). Telomeres are nucleoprotein structures that cap the end of chromosomes and thereby protect against unwanted recombination and degradation (Citation16). Telomeres consist of DNA repeated TTAGGG sequences, that shorten after each cellular division. The GGG sequence of telomere is particularly sensitive to oxidative damage by ROS and is the main cause of accelerated telomere length (TL) shortening. Activation of RAAS, in particular Ang II-mediated oxidative DNA damage, accelerates TL shortening (Citation17,Citation18). In the Framingham Heart Study, the relationship between circulating RAAS determinants and TL was investigated. Renin and TL were found to be significantly inversely correlated, while aldosterone and TL were significantly positively correlated (Citation19). Recent studies have shown that TL of newborns is influenced by intrauterine exposures to oxidative stress, whereas TL of newborns is associated with the prediction of lifespan (Citation20). Excessive maternal TL shortening is an index of senescence causing genomic instability, increased the risk of age-related diseases and neural-crest cell-related birth defects (Citation21,Citation22). It has been suggested that TL is a long-term biomarker of chronic oxidative stress (Citation21,Citation23). From this background, we hypothesize that an impaired hemodynamic adaptation to pregnancy in the first-trimester increases maternal RAAS activation and subsequent oxidative stress exposure resulting in maternal TL shortening. In addition, because ovaria stimulation excessively increases the activation of RAAS (Citation11), maternal TL shortening will be accelerated in these women. From this background, we aim to study associations between RAAS activation and TL in women after natural and in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) conceived pregnancies.
Materials and methods
Study design
We used data from a subcohort embedded in the ongoing prospective Rotterdam periconception cohort (Predict Study) at the Department of Obstetrics and Gynecology of the Erasmus MC, University Medical Center Rotterdam, the Netherlands (Citation24). This subcohort was designed to study the (patho)physiology of early placental development (VIRTUAL Placenta study). The VIRTUAL Placenta study enrolled 241 women between January 2017 and March 2018 (Citation25). This study population conceived either natural, through IVF or ICSI. Inclusion criteria are a minimum maternal age of 18 years or older, with an ongoing intrauterine singleton pregnancy of less than 10 weeks of gestation and familiar with the spoken and written Dutch language. Women are excluded from analysis in case of oocyte donation, twin pregnancies, fetuses or neonates with congenital malformations, miscarriages, and withdrawal from the study. We excluded women without measurements of RAAS determinants and missing TL assessment.
Naturally conceived pregnancies were dated based on the first day of the last menstrual period with a regular cycle between >25 and <35 days. In IVF/ICSI pregnancies, GA was calculated from oocyte retrieval day plus 14 days or, for cryopreserved embryo transfer, from the transfer day plus 19 days. Gestational age was estimated using crown – rump length (CRL) in pregnancies with irregular cycles, when the last menstrual period was unknown, or when gestational age based on last menstrual period differed by more than 6 days from the calculated gestational age based on CRL. Maternal characteristics, medical and obstetrical history and lifestyle behaviors were obtained from a self-reported questionnaire and a personal interview at the study. Entry visit maternal blood pressure, weight, and height were standardized and measured at the same visit. Written informed consent was obtained from all study participants during enrollment. Venous blood samples were drawn from all mothers at 9 and 20 weeks of GA. The study protocol was approved by The Central Committee on Research involving Human Subjects and the institutional review boards of all participating hospitals (15 October 2009; MEC-2004-227). Written informed consent was obtained from all study participants at enrollment.
Renin, prorenin, and aldosterone measurement
To determine the association between RAAS activation and TL length, we measured the following RAAS determinants: renin, prorenin, and aldosterone. As described in Wiegel et al (Citation11), non-fasting venous blood samples were taken at 9 weeks of gestation at the Erasmus MC, University Medical Center Rotterdam, the Netherlands. Blood was collected (January 2017 until March 2018) in 10-mL vacutainer ethylenediaminetetraacetic acid (EDTA) tubes and centrifuged (2000 g for 10 min). Plasma was stored at −80°C in macro-tubes until analysis (in April 2019). Renin and prorenin concentrations were measured by an immunoradiometric assay (Cisbio, Saclay, France) making use of an active site-directed radiolabeled antibody that recognizes renin only (sensitivity 1 pg/ml, interassay variability 4%) (Citation26). Prorenin concentrations were calculated by subtracting renin from total renin measured after activating prorenin with aliskiren (Citation27). Aldosterone concentrations were measured by solid-phase radioimmunoassay (Demeditec Diagnostics, Kiel, Germany; sensitivity 12 pg/ml, interassay variability 5%).
DNA-isolation and telomere length measurement
Genomic DNA from mothers was extracted from EDTA blood samples, drawn at 20 weeks of GA, with the Reliaprep kit (Promega, Leiden, the Netherlands) on a Tecan Evo robot (Tecan Trading Aargua, Switzerland). DNA concentrations were measured with the Nanodrop (ThermoFisher, Waltham, United States of America) and normalized to 50 ng/ul.
Relative TL (also TS ratio) was measured using a qPCR assay based on the method described by Cawthon (Citation28) with minor modifications. TS ratio is the relative amount of telomeric DNA (T) to the beta-globin single copy gene (S). For each sample, the telomere and 36B4 assay were run in the same well position but in different 384 wells PCR plates. Each reaction contained 2 ng DNA, 1 uM of each of the telomere primers (tel1b-forward: GGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT, tel2b-reverse: GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCTT) or 250 nM of the 34B4 primers (36B4u-forward: CAGCAAGTGGGAAGGTGTAATCC, 36B4d-reverse: CCCATTCTATCATCAACGGGTACAA) and 1× Quantifast SYBR green PCR Mastermix (Qiagen, Hilden, Germany). The reactions for both assays were performed in duplicate for each sample in a QuantStudio Flex 7 real time PCR machine (Applied Biosystems, Waltham, United States of America). The cycle threshold (Ct) values and PCR efficiencies were calculated per plate using the MINER algorithm (Citation29). Duplicate Ct values with a Coefficient of Variance (CV) of more than 1% were repeated a second time in a different run. The average Ct values (of the duplicate measurements) per sample were adjusted for PCR efficiency using the formula Q = 1/(1+PCR eff)^Ct. The TS ratio was calculated by dividing the Q of the telomere assay by the Q of the 34B4 assay. Each 384 wells PCR plate contained a set of 7 control samples. The average TS ratio of these 7 samples was used to normalize for plate batch effects. To validate the measured TS ratios, 29 random samples were run twice and the CV of that experiment was 4.6%, with an r = 0.87. TS ratio will be indicated as TL in results.
Statistical analysis
Descriptive statistics of the study population at baseline are presented as mean and standard deviation for normal distributed variables and median and interquartile range for skewed variables. Frequencies (proportions), were used for categorical variables. In Supplements table S1 data was stratified for mode of conception and to test for differences the Student’s t-test for normally distributed data, Kruskall-Wallis test for non-normally distributed data and the Chi-squared test for categorical data were used (Citation30). First, because of positively skewed distributions, RAAS component concentrations were transformed using a natural logarithmic transformation to obtain an approximately normal-distribution. Spearman correlations were used to evaluate the crude correlations between RAAS determinants, and thereby activation, i.e., renin, prorenin, aldosterone, and maternal TL. To assess the associations between RAAS concentrations and maternal TL (T/S ratio) we used multivariable linear regression. First, we estimated the effect of the RAAS determinants using a crude model. Thereafter, the model was adjusted for maternal age, as this is a known confounder for TL (model 1). In a second model, we additionally adjusted for conception mode, first-trimester body-mass index (BMI), mean arterial blood pressure (MAP), and smoking status (model 2). To assess the distribution of maternal TL and RAAS activation in the natural conceived pregnancy group compared to the IVF/ICSI group box-and-whisker plots were generated and differences were compared by Mann-Whitney-U-test. All analyses were performed in R (R for Windows, version 3.5; R Core Team). P-values <0.05 were considered statistically significant.
Results
The VIRTUAL placenta study included 241 pregnancies. Pregnancies were excluded because of miscarriage (n = 22) or withdrawal from the study (n = 1). From the 218 ongoing pregnancies, pregnancies were excluded if there was no first- or second trimester blood sample available (n = 61), conception by oocyte donation (n = 4) or a fetus with a congenital malformation (n = 8). A total of 145 pregnancies were selected and included for analysis.
Baseline characteristics of the study population are shown in . The mean maternal age was 32.4 (SD: 4.5) years. From the remaining 145 pregnancies a total of 84 (57.9%) conceived naturally and 61 (42.1%) were conceived through IVF/ICSI treatment. The majority of our study population was from a Dutch geographic origin (n = 114, 80.9%) and highly educated (n = 83, 58.9%). The average BMI at study entry was 24.9 (IQR: 22.38; 27.99). The MAP was 80 mmHg (IQR: 73; 87). Periconceptional folic acid supplements were used in 82.8% of the study population. The percentage of women consuming alcohol in the preconception period was 26.9%, while 11.0% of the women smoked during the preconception period. After stratification for mode of conception, we found significant more nulliparous in the natural conceived pregnancy group versus the IVF/ICSI group. Thereby folic acid supplement use was significantly lower in the natural conceived pregnancy group compared to the IVF/ICSI group. No further significant differences were found between both groups.
Table 1. Baseline characteristics of the virtual placenta study population.
The correlations by Spearman rank test between maternal renin, prorenin and aldosterone concentrations at 9 weeks gestation and TL (T/S ratio) are displayed in . Renin concentrations revealed a significant inverse correlation with TL (, R = −0.27; p = 0.013). There was an inverse correlation between prorenin concentrations and TL, albeit not significant (, R=−021; p = 0.06). No correlation between aldosterone concentrations and TL was found (). Given the relatively small effect size (R < 0.40), it can be inferred that potential confounders other than renin, such as maternal age, play a significant role in determining TL. Hence, in two models the adjusted linear associations were performed.
Figure 1. Correlations by Spearman Rank test between maternal telomere length (T/S ratio) and a) renin, b) prorenin, c) aldosterone.
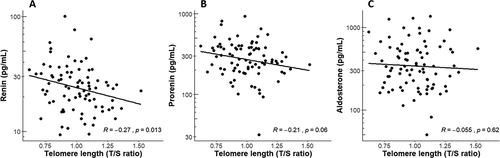
shows the results of the multivariable linear regression analyses with the associations between renin, prorenin and aldosterone, and maternal TL in the total study population. A significantly negative association was found between renin concentrations and maternal TL (crude β: −0.094 [95% CI: −0.17, −0.01], p = 0.02). After adjustment for maternal age and additional adjustment for mode of conception, BMI, MAP and smoking status, the association remained significant (Model 1 β: −0.092 [95% CI: −0.17, −0.01], p = 0.025 and Model 2 β: −0.086 [95% CI: −0.17, −0.00], p = 0.047), respectively. Prorenin concentrations tended to show a negative association with maternal TL (crude β: −0.07 [95% CI: −0.14, −0.003], p = 0.06, Model 1 β: −0.065 [95% CI: −0.14, −0.01], p = 0.079 and Model 2 β: −0.058 [95% CI: −0.13, − 0.02], p = 0.14), albeit not significant. We did not observe an association between aldosterone concentrations and maternal TL, nor after adjustment.
Table 2. The associations between first-trimester log-transformed RAAS component concentrations at week 9 of pregnancy and telomere length at 20 weeks of pregnancy.
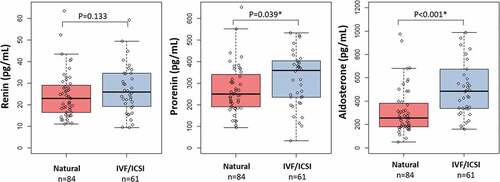
In addition, we performed a secondary analysis focusing on the association between mode of conception, in particular natural (n = 84) and IVF/ICSI (n = 61). We observed that maternal TL was significantly shorter, p = 0.04, in the IVF/ICSI group (TL, mean±SD: 0.96 ± 0.17), compared to maternal TL in the natural group (TL, mean±SD: 1.02 ± 0.18), as shown in . We also observed increased renin, prorenin and aldosterone concentrations in the IVF/ICSI group compared to the natural group (). However, after adjusting the multivariable linear regression analysis for mode of conception, the negative association between renin and maternal TL remained significant ().
Figure 2. Box-and-whisker plot demonstrating the distribution of telomere length (TS ratio) in the natural conceived group compared with the IVF/ICSI group. Boxplots present median, 10th, 25th, 75th, and 90th percentile. Telomere length was compared by Mann-Whitney U test. *Significance at p < 0.05.
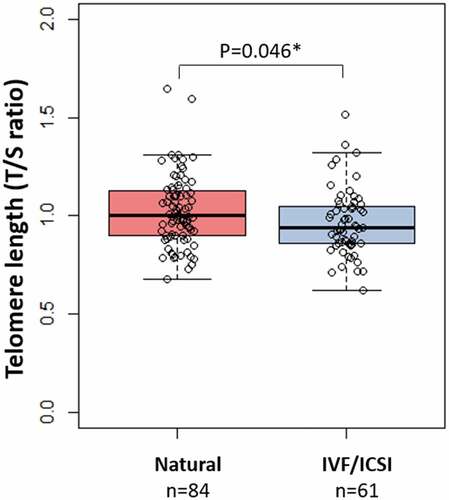
Figure 3. Box-and-whisker plot demonstrating the distribution of renin, prorenin and aldosterone concentrations in the natural conceived group compared with the IVF/ICSI group. Boxplots present median, 10th, 25th, 75th, and 90th percentile. Renin, prorenin and aldosterone concentrations were compared by Mann-Whitney U test. *Significance at p < 0.05.
Discussion
To our knowledge, this is the first study showing associations between first-trimester maternal RAAS activation and maternal TL. Higher renin concentrations show significantly shorter maternal TL, supporting our hypothesis that impaired hemodynamic adaptations increase first trimester activation of RAAS resulting in excessive oxidative stress exposure and subsequent shortening of maternal TL. Prorenin concentrations also tend to show a negative association with maternal TL. However, no associations were found between aldosterone concentrations and maternal TL. Moreover, a significantly shorter maternal TL was found in pregnancies conceived after IVF/ICSI treatment compared to maternal TL of naturally conceived pregnancies.
The identification of TL as a stable biomarker of the first-trimester chronic oxidative stress status in women, due to an adverse periconceptional cardiovascular status, is of great interest. Because cardiovascular-related adverse pregnancy outcomes originate in this specific period and subsequent impact maternal and pregnancy outcomes as well as their cardiovascular health across the life course. Efforts for early prediction and prevention should be pursued.
The found significantly inverse association between maternal TL and renin concentrations corresponds with results of the Framingham Heart Study, described by Vasan et al (Citation31). They found that TL, in general, was significantly inversely related to renin and directly to aldosterone. On the contrary, Benetos et al. described an inverse correlation between TL and plasma aldosterone (Citation32). According to our hypothesis, pathologically elevated concentrations of aldosterone would induce ROS production and thereby accelerating telomere attrition. In our study population no significant association was found between aldosterone concentrations and TL, possibly due to a smaller study population. Also, our study population consisted of only pregnant women, whereas Vasan et al. studied both men and women with ages between 48 and 73 years and Benetos et al. studied only men between 43 and 68 years (Citation33). It is also possible that the primarily vasoactive arm (reflected as prorenin- and renin concentrations) has more influence on telomere attrition due to causing more oxidative stress and inflammation than the primarily sodium regulating arm (reflected as aldosterone concentrations). Or that the role of aldosterone, as an inducer of ROS production, in pregnancy may not be fully identical to that in the general population. As the new increased steady state of aldosterone in pregnancy, responsible for maintaining the homeostasis, is accepted as normal (Citation34). Furthermore, it is likely that the associations between TL and RAAS activation is predominantly observed with renin, as prorenin is an inactive prohormone of which the exact physiological role during pregnancy has not yet been elucidated. Renin, on the other hand, serves as the active metabolite responsible for catalyzing the conversion of angiotensinogen into Ang I.
As described earlier, increased Ang II, as a consequence of adversely activated RAAS, has been shown to be of major impact in initiating and sustaining several mechanisms that contribute to increased formation of oxidative stress. In the vessels, even a local positive-feedback mechanism is established for oxidative stress and inflammation, knowing that inflammatory cells release enzymes that generate Ang II (Citation35). Our findings correspond with above, showing that inadequate increased Ang II (in our study reflected as renin concentrations) possibly results in excessive oxidative stress and inflammation expressed by shorter maternal TL.
TL is suggested to be a long-term biomarker of chronic oxidative stress, as it shows the cumulative burden of oxidative stress (Citation23). Short telomeres and telomere dysfunction, independently of age have been linked to numerous age-related diseases. Large population-based studies identify that subjects with shorter telomeres were characterized by a significantly higher hazard ratio for all-cause mortality compared to those with higher TL (Citation36). Recently, it has been hypothesized that periconceptional long-term exposure to excessive oxidative stress and inflammation accelerates maternal TL shortening, and thereby increasing the underlying risk of neural tube defects in offspring (Citation21). In this manner, shorter maternal TL were also associated with an increased risk of having a child with a ventricular septal defect (Citation22). As the vulnerability of telomeres for oxidative stress and inflammation is well known, the RAAS is suggested to contribute to the acceleration of TL shortening. RAAS contributes to the pathogenesis of several human diseases that have a clear association with accelerated TL shortening, including cardiovascular diseases, stroke, and diabetes (Citation37). By this means, several studies confirmed that Ang II induces the shorting of TL, in particular accelerated the rate of telomere loss (>2-fold versus control) in a dose-dependent manner (Citation17,Citation18,Citation38). This is consistent with our findings that a higher level of renin is associated with significant shorter TL due to excessive exposure to oxidative stress.
We found significantly shorter maternal TL in pregnancies conceived through IVF/ICSI. A possible explanation for this result is the influence of extra-renal RAAS components, like prorenin released from the ovaries (Citation39). Wiegel et al. previously described the influence of ovarian stimulation, in particular the stimulating role of the corpus luteum, on maternal RAAS activation. Thereby, showing that the ovarian role, depending on hormone used, can result in significantly higher RAAS determinants activation during pregnancy and in this way contribute to excessive oxidative stress and inflammation (Citation11). Alternatively, taking into consideration that TL has been described as a powerful biomarker for aging and aging-associated pathology (Citation40), it might be that women with shorter TL exhibit a more advanced biological age before the onset of pregnancy compared to women of identical calendar age, and thereby requiring IVF/ICSI treatment. However, after adjusting the linear regression analysis for mode of conception, the negative association between renin and maternal TL remained significant. This indicates that the potential effect of ovarian stimulation cannot entirely explain our findings.
The main strength of the study is the unique data of the RAAS determinants, representing RAAS activation during the first trimester period, in relation to maternal TL. Our results give insight into the consequence of inadequate activation of RAAS and thereby cardiovascular maladaptation to pregnancy, resulting in excessive exposure to oxidative stress and inflammation and shortening of TL, even in this young group of women. The single centered setting limits variability, where standardized protocols were used for the outcome of measurements. Thereby, the multivariable analyses were adjusted for multiple confounders.
Possible limitations of our study are the measurement of TL, concerning that mean TL is measured, while cell senescence seems to be related to the shortest TL per cell (Citation41). Single measurements of the RAAS determinants represent the current status of maternal RAAS activation, whereas TL is stated to be a long-term biomarker. Furthermore, Ang II was not measured despite its major role in increasing oxidative stress and inflammation. It must be noted that it is unlikely that our study will be replicated with inclusion of Ang II, given the great difficulty of measuring Ang II adequately.
Additionally, the tertiary setting of our study population limits the generalizability of our study results. Although we showed a statistically significant correlation between maternal renin concentrations and TL, the effect size of the correlation is relatively small, suggesting the involvement of other factors which has been investigated by the linear models. However, residual confounding cannot be excluded due to the observational nature of our cohort.
The scientific implications of the associations between RAAS and TL will renew the interest in both potential biomarkers as potential early predictors of adverse cardiovascular related maternal and pregnancy outcome applicability. At this moment, the clinical implications are limited although clinicians might be more alert about patient conditions, such as age and IVF/ICSI treatments, and behaviors, such as lifestyle, and the influence on RAAS. Measurements of the RAAS determinants activation, including Ang II, and maternal TL in the preconception period, first, second, and third trimester, and non-pregnant women as a control group, could be of great interest in giving more insight into the association between maternal RAAS and TL during pregnancy. Taking into account that other associations between maternal TL and pregnancy outcome have been reported. Shorter TL and in particular decreased or absent telomerase activity, have been found in placentas of fetal growth restricted newborns (Citation42). Moreover, our findings contribute to the recognition of the importance of a life course approach in women’s and offspring’s cardiovascular health. Improving lifestyle and health conditions in women contemplating pregnancy and, in that way, reducing exposure to excessive oxidative stress is of great importance. There could be a role for the use of E-health intervention platforms, such as the program www.SmarterPregnancy.co.uk for periconception and pregnancy care (Citation43).
Conclusion
Our findings support the hypothesis that impaired hemodynamic adaptations to pregnancy resulting in a chronic and excessive activation of RAAS is associated with a shortening of maternal TL. However, the causality has to be shown in a longitudinal study starting preconceptionally up and until delivery. Negative associations independent from maternal age were found between renin concentrations and maternal TL in natural and IVF/ICSI conceived pregnancies. Therefore, RAAS determinants activation during the first trimester of pregnancy is suggested to contribute to TL shortening. To confirm our findings and investigate whether maternal TL could serve as an early biomarker of the hemodynamic adaptations to pregnancy and subsequent adverse pregnancy outcomes, more research is needed.
Supplemental Material
Download MS Word (16.7 KB)Acknowledgments
The authors wish to thank Pascal. P. Arp from the Department of Internal Medicine, Erasmus MC, University Medical Centre Rotterdam, the Netherlands, for executing the telomere length measurements. The authors wish to gratefully acknowledge the Rotterdam Periconceptional Cohort team for data acquisition and thank the participants for their contributions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, DAF, upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2238086
Additional information
Funding
References
- West CA, Sasser JM, Baylis C. The enigma of continual plasma volume expansion in pregnancy: critical role of the renin-angiotensin-aldosterone system. Am J Physiol Renal Physiol. 2016 Dec 1;311(6):F1125–F1134.
- Steegers-Theunissen RP, Twigt J, Pestinger V, et al. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013 Nov;19(6):640–9.
- Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998 Dec;54(6):2056–2063.
- Campbell DM, MacGillivray I. Comparison of maternal response in first and second pregnancies in relation to baby weight. BJOG: An Int J Obstetrics & Gynaecol. 1972;79(8):684–693. doi: 10.1111/j.1471-0528.1972.tb12901.x
- Gibson HM. Plasma volume and glomerular filtration rate in pregnancy and their relation to differences in fetal growth. J Obstet Gynaecol Br Commonw. 1973 Dec;80(12):1067–1074. doi: 10.1111/j.1471-0528.1973.tb02981.x
- Salas SP, Rosso P. Plasma volume, renal function, and hormonal levels in pregnant women with idiopathic fetal growth restriction or preeclampsia [Article]. Hypertens Pregnancy. 1998;17(1):69–79. doi: 10.3109/10641959809072239
- Salas SP, Rosso P, Espinoza R, et al. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation [Article]. Obstet Gynecol. 1993;81(6):1029–1033.
- Rosso P, Donoso E, Braun S, et al. Maternal hemodynamic adjustments in idiopathic fetal growth retardation. Gynecol Obstet Invest. 1993;35(3):162–165. doi: 10.1159/000292690
- Irani RA, Xia Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta. 2008 Sep;29(9):763–771. doi: 10.1016/j.placenta.2008.06.011
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, et al. Physiological changes in pregnancy. Cardiovasc J Afr. 2016 Mar;27(2):89–94.
- Wiegel RE, Jan Danser AH, Steegers-Theunissen RPM, et al. Determinants of maternal renin-angiotensin-aldosterone-system activation in early pregnancy: Insights from 2 cohorts. J Clin Endocrinol Metab. 2020;105(11):3505–3517. doi: 10.1210/clinem/dgaa582
- Sowers JR. Hypertension, angiotensin II, and oxidative stress [Editorial]. New Engl J Med. 2002;346(25):1999–2001. doi: 10.1056/NEJMe020054
- Fanelli C, Zatz R. Linking oxidative stress, the renin-angiotensin system, and hypertension [Editorial]. Hypertension. 2011;57(3):373–374. doi: 10.1161/HYPERTENSIONAHA.110.167775
- Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin-angiotensin system and cardiovascular risk – Authors reply. Lancet. 2007;370(9581):24–25. doi: 10.1016/S0140-6736(07)61036-8
- Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011 Feb 17;470(7334):359–365.
- Martens DS, Plusquin M, Gyselaers W, et al. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016 Oct 18;14(1):148.
- Herbert KE, Mistry Y, Hastings R, et al. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008 Feb 1;102(2):201–208.
- Feng X, Wang L, Li Y. Change of telomere length in angiotensin II-induced human glomerular mesangial cell senescence and the protective role of losartan. Mol Med Rep. 2011;4(2):255–260. doi: 10.3892/mmr.2011.436
- Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: The Framingham heart study. Circulation. 2008;117(9):1138–1144. doi: 10.1161/CIRCULATIONAHA.107.731794
- Bijnens EM, Zeegers MP, Derom C, et al. Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med. 2017 Nov 21;15(1):205.
- Aoulad Fares D, Schalekamp-Timmermans S, Nawrot TS, et al. Preconception telomere length as a novel maternal biomarker to assess the risk of spina bifida in the offspring. Birth Defects Res. 2020 May 15;112(9):645–651.
- Fares D, Wiegel R, Eggink A, et al. Shorter periconception maternal telomere length and the risk of congenital cardiac outflow defects in the offspring. Eur J Clin Invest. 2022;52(8):e13784.
- Babizhayev MA, Savel’yeva EL, Moskvina SN, et al. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am J Ther. 2011 Nov;18(6):e209–26.
- Steegers-Theunissen RP, Verheijden-Paulissen JJ, van Uitert EM, et al. Cohort profile: The rotterdam periconceptional cohort (predict study). Int J Epidemiol. 2016 Apr;45(2):374–381.
- Reijnders IF, Mulders A, Koster MPH, et al. First‐trimester maternal haemodynamic adaptation to pregnancy and placental, embryonic and fetal development: The prospective observational Rotterdam Periconception cohort. BJOG: An Int J Obstetrics & Gynaecol. 2022;129(5):785–795. doi: 10.1111/1471-0528.16979
- Campbell DJ, Nussberger J, Stowasser M, et al. Activity assays and immunoassays for plasma Renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem. 2009 May;55(5):867–877.
- Batenburg WW, de Bruin RJ, van Gool JM, et al. Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008 Jun;28(6):1151–1157.
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009 Feb;37(3):e21. doi: 10.1093/nar/gkn1027
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005 Oct;12(8):1047–1064. doi: 10.1089/cmb.2005.12.1047
- Aoulad Fares D. Table 3. Baseline characteristics of the Virtual Placenta study population stratified for mode of conception. 2022.
- Vasan RS, Demissie S, Cupples LA, et al. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: The framingham heart study response [Letter]. Circulation. 2008 Nov;118(19):E689–E689.
- Benetos A, Gardner JP, Kimura M, et al. Aldosterone and telomere length in white blood cells. J Gerontol A Biol Sci Med Sci. 2005 Dec;60(12):1593–1596.
- Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with echocardiographic left ventricular mass the framingham heart study. Circulation. 2009 Sep;120(13):1195–1202.
- Escher G. Hyperaldosteronism in pregnancy. Ther Adv Cardiovasc Dis. 2009 Apr;3(2):123–132. doi: 10.1177/1753944708100180
- Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001 Apr;37(4):1047–1052. doi: 10.1161/01.HYP.37.4.1047
- Mons U, Muezzinler A, Schottker B, et al. Leukocyte telomere length and all-cause, cardiovascular disease, and cancer mortality: Results from individual-participant-data meta-analysis of 2 large prospective cohort studies. Am J Epidemiol. 2017 Jun 15;185(12):1317–1326.
- Abadir PM. The frail renin-angiotensin system [review]. Clin Geriatr Med. 2011;27(1):53–65. doi: 10.1016/j.cger.2010.08.004
- Mogi M. Effect of renin–angiotensin system on senescence [review]. Geriatr Gerontol Int. 2020;20(6):520–525. doi: 10.1111/ggi.13927
- Itskovitz J, Sealey JE. Ovarian prorenin-renin-angiotensin system. Obstet Gynecol Surv. 1987 Sep;42(9):545–551. doi: 10.1097/00006254-198709000-00003
- Fasching CL. Telomere length measurement as a clinical biomarker of aging and disease. Crit Rev Clin Lab Sci. 2018 Nov;55(7):443–465. doi: 10.1080/10408363.2018.1504274
- Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan [Review]. Prog Biophys Mol Biol. 2011 Jul;106(1):323–336. doi: 10.1016/j.pbiomolbio.2010.12.005
- Fragkiadaki P, Tsoukalas D, Fragkiadoulaki I, et al. Telomerase activity in pregnancy complications (Review). Mol Med Rep. 2016 Jul;14(1):16–21.
- Oostingh EC, Ophuis RH, Koster MP, et al. Mobile health coaching on nutrition and lifestyle behaviors for subfertile couples using the smarter pregnancy program: Model-based cost-effectiveness analysis. JMIR mHealth uHealth. 2019 Oct 23;7(10):e13935.

