?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Aims
To objectively study cardiorespiratory fitness (CRF) and physical activity (PA) and to evaluate limiting factors of exercise intolerance associated with poor CRF after severe pre-eclampsia.
Methods
In this single-centre, cross-sectional study, CRF was measured as peak oxygen uptake (VO2peak) during a cardiopulmonary exercise test (CPET) on a treadmill in women 7 years after severe pre-eclampsia. Ninety-six patients and 65 controls were eligible to participate. Cardiac output (CO) was measured by impedance cardiography. PA was measured using accelerometers.
Results
In 62 patients and 35 controls (mean age 40 ± 3 years), the VO2peak (in mL·kg–1·min–1) values were 31.4 ± 7.2 and 39.1 ± 5.4, respectively (p<0.01). In the patients, the COpeak was (9.6 L·min-1), 16% lower compared to controls (p<0.01). Twelve patients (19%) had a cardiac limitation to CPET. Twenty-three (37%) patients and one (3%) control were classed as unfit, with no cardiopulmonary limitations. The patients demonstrated 25% lower PA level (in counts per minute; p<0.01) and 14% more time being sedentary (p<0.01), compared with the controls. Twenty-one patients (34%) compared with four (17%) controls did not meet the World Health Organization’s recommendations for PA (p=0.02). Body mass index and PA level accounted for 65% of the variability in VO2peak.
Conclusion
Significantly lower CRF and PA levels were found in patients on long-term follow-up after severe pre-eclampsia. CPET identified cardiovascular limitations in one third of patients. One third appeared unfit, with adiposity and lower PA levels. These findings highlight the need for clinical follow-up and exercise interventions after severe pre-eclampsia.
Introduction
Preeclampsia occurs in up to 4% of all pregnancies in the industrialized world (Citation1). Women with a history of preeclampsia have an increased risk of cardiovascular disease (CVD) later in life (Citation2,Citation3). Studies suggest the CVD risk profile to be different in two different subgroups of preeclampsia, i.e., early-onset preeclampsia (onset before 34 weeks of gestation) and late-onset preeclampsia (onset at or after 34 weeks of gestation) (Citation4). Systematic reviews demonstrate an approximately doubled risk of ischemic heart disease, cerebrovascular incidents, and CVD mortality after preeclampsia (Citation5). Preeclampsia with severe features in the clinical presentation (severe hypertension, thrombocytopenia, impaired liver function, renal insufficiency, pulmonary edema, severe new-onset headache) (Citation6) influence the long-term outcome, and seem to double the odds risk of future CVD (Citation7). Observational echocardiographic studies demonstrate reduced left ventricular (LV) diastolic and systolic function at long-term follow-up in women after previous preeclampsia (Citation8,Citation9).
Cardiorespiratory fitness (CRF) refers to the capability of the circulatory and respiratory systems to supply oxygen to skeletal muscle mitochondria for energy production needed during sustained physical activity (PA). CRF is an important marker of physical fitness and correlates well with overall health status. A cardiopulmonary exercise test (CPET) can express CRF and is the gold-standard measurement of peak oxygen uptake (VO2peak) and gas exchange variables during incremental exercise (Citation10,Citation11). To our knowledge, only one study has characterized CRF after preeclampsia at long-term follow-up by a CPET (Citation12).
We hypothesized that women with a history of severe preeclampsia would have poor CRF and low PA levels compared with healthy controls. Accordingly, the primary aim of this study was to characterize CRF and PA levels in women after severe preeclampsia, compared with healthy controls. The secondary aims were to evaluate limiting factors of poor CRF, including the evaluation of LV function by echocardiography.
Materials and methods
Design and study population
This cross-sectional, single-center study was conducted at Oslo University Hospital from October 2013 to January 2016. After a review of the hospital’s database, women diagnosed with severe preeclampsia between 2005 and 2010, defined as the index pregnancy, were invited to participate. Inclusion criteria for the patient group were previous severe preeclampsia with early and late-onset disease and giving birth at the Department of Obstetrics at Oslo University Hospital, Rikshospitalet. The control group consisted of women randomly recruited from a database of women with previous healthy pregnancies giving birth at the same hospital between 2008 and 2009. The inclusion criteria for the control population were earlier healthy pregnancies. Exclusion criteria for the patient and the control group were any physical inability to perform CPET, ongoing pregnancy, breastfeeding, assisted reproductive technology therapy, neoplastic disease therapy, or debilitating psychiatric illness.
Data from the pregnancy and clinical follow-up of the study populations, including the patient and controls groups, have recently been published (Citation9).
The study was approved by the Norwegian Regional Committee for Medical and Health Research Ethics (REK Southeast, No. 2013-585b) and the local institutional board at Oslo University Hospital. Written informed consent was obtained from all study participants following the Declaration of Helsinki (Citation13).
Patient and public involvement
This research was approved by the Data Protection Officer at Oslo University Hospital, required to safeguard the research participant’s privacy, interest, and rights.
Clinical characteristics and registrations
Severe preeclampsia was defined as a new onset of severe hypertension, with systolic blood pressure (SBP) ≥ 160 mmHg or diastolic blood pressure (DBP) ≥ 110 mmHg, and one or more severe features (thrombocytopenia, impaired liver function, renal insufficiency, pulmonary edema, or severe new-onset headache) (Citation6). The diagnosis of preeclampsia included previous criteria from The International Society for the Study of Hypertension in Pregnancy (ISSHP), including proteinuria defined as ≥30 mg∙mmol−1 albumin in a urine spot sample from a creatinine assay or ≥ 1+ on a repeat dipstick test (Citation14). Early-onset preeclampsia was defined as occurring at <34 weeks of gestation and late-onset at ≥34 weeks of gestation, according to the previous ISSHP guidelines (Citation14). Maternal and fetal data were obtained through a standardized participant interview and review of the medical records. Any family history of CVD was defined from reporting it in first-degree relatives. Hypertension was defined as any use of antihypertensive medication.
Measurements
Clinical tests, blood sampling, pulmonary function, CPET, and questionnaires were undertaken on two days. In addition, objective measures of PA were registered. All tests were performed by the same physician (LG) and an exercise physiologist.
Echocardiography
Echocardiography was performed using an ultrasound scanner (Vivid E9, GE, Horten, Norway) 3–6 months before CPET. EchoPac version 13.1 (GE, Horten, Norway) was used for analysis. LV end-diastolic volume and ejection fraction (EF) were calculated using Simpson’s modified biplane method (Citation15). The LV mass was calculated from LV dimensions (Citation16). The mitral peak early (E), late (A) diastolic flow, and peak early (e´) velocities were measured (Citation17). LV global longitudinal strain (GLS) was measured by two-dimensional speckle tracking echocardiography.
Pulmonary function tests
Pulmonary function was assessed by spirometry, maximal voluntary ventilation, and the diffusing capacity of the lungs for carbon monoxide (DLco) (Viasys, Wurzburg, Germany) following the recommendations of the American Thoracic Society/European Respiratory Society task force (Citation18,Citation19).
Cardiopulmonary exercise testing
CPET was performed on a treadmill (Woodway PPS Med, Germany) using a stepwise modified Balke protocol until exhaustion (Citation20). Gas exchange and exhaled volumes were measured directly, breath by breath (Vyntus CPX Metabolic Cart, CareFusion Corporation, Hochberg, Germany). Blood pressure (BP) was measured at rest, during and after CPET (Tango Stress Test Monitor, SunTech Medical Instruments, Morrisville, NC, USA). Heart rate (HR) was recorded, and a 12-lead electrocardiogram (ECG) (Custo cardio 100, CustoMed, Ottobrunn, Germany) was used. Percutaneous oxygen saturation was measured by finger pulse oximetry (NONIN 8600, Medical Inc., Minneapolis, MN, USA). The rating of perceived exertion (Citation21) was assessed using the Borg scale6–20 (Citation22). Post-exercise capillary blood lactate and hemoglobin concentrations (ABL 700 series, Radiometer, Copenhagen, Denmark) were measured within 60 s after exercise (Citation23). Before each CPET, a complete volume and gas calibration was performed, and all underwent treadmill familiarization before starting.
Cardiac output monitoring
Stroke volume (SV), HR, and cardiac output (CO) were monitored continuously at rest and throughout the CPET by impedance cardiography with an integrated 6-lead electrocardiogram using the PhysioFlow Q-link device (Manatec Biomedical, Paris, France). This technology applies the cyclic variations in transthoracic impedance during the cardiac cycle to estimate SV and has been validated against the direct Fick method (Citation24,Citation25).
Physical activity assessment
For the objective assessment of PA, all participants wore an accelerometer (ActiGraph GT1M, LLC, Pensacola, FL, USA) on their right hip for seven consecutive days. Accelerometer data were extracted from the vertical axis records in 10-s epochs and were reanalyzed to produce PA and sedentary time variables using KineSoft (version 3.3.20, Saskatchewan, Canada; http://www.kinesoft.org). Data were included in the analysis if the subject had a least 10 hours of valid activity recordings per day, for at least 4 days (Citation26). Steps per day, count per minute, sedentary time, and time spent in moderate to vigorous physical activity (MVPA) were registered.
Data handling
Being overweight was defined as a body mass index (BMI) ≥ 25 kg∙m−2, and obesity was defined as BMI ≥30 kg∙m−2, according to the World Health Organization (WHO) classification (Citation27). The predicted values for spirometry were calculated using the Global Lung Function Initiative equations (Citation28,Citation29). The highest VO2 sampled over 30 s was defined as VO2peak. Reference values for VO2peak values were used from a large Norwegian population of 759 healthy adults who successfully completed CPET using the same treadmill protocol as in the present study (Citation20). Low CRF was defined as VO2peak <85% of that predicted (Citation30). Ventilatory threshold was determined by the ventilatory equivalent method. A ventilatory limitation was defined as a breathing reserve ≤ 15% or 11 L∙min−1, and a gas exchange limitation was defined as a VE/VCO2 slope ≥ 34 (Citation31). CPET end criteria defining maximal effort were respiratory exchange ratio (RER ≥1.10) or RPE on Borg scale6–20 ≥17 (Citation22).
Cardiac limitation was defined as an oxygen pulse < 80% of the predicted, an HRpeak <90% of the age-predicted HRpeak, and an HR reserve < 15 beats/min in the absence of pulmonary limitations (Citation32). Hypertensive response to exercise (HRE) was defined as SBPpeak >196 during maximal effort (Citation33). Deconditioning was defined as poor VO2peak (VO2peak <85% of expected) in the absence of cardiac or pulmonary limitations. Participants were classified as physically active if they performed ≥150 min of moderate to vigorous PA per week in ≥ 10-min bouts (Citation34).
Statistical analysis
Standard statistical analyses were performed using IBM SPSS Statistics (v. 25; IBM Corp., Armonk, NY, USA). Results are presented as the mean ± standard deviation of the mean (SD). Differences between patients and controls and between groups of early vs late-onset preeclampsia were analyzed using Student’s t test and ANOVA for normally distributed data. The Mann – Whitney nonparametric U test was used for non-normally distributed data. Categorical variables between the groups were compared using the chi-squared test or Fisher’s exact test as appropriate. Simple linear regression analyses were used to analyze any relationship between all relevant CPET variables (independent variables) and VO2peak (dependent variable). Linearity was assessed by partial regression plots. Normality was assessed by Q–Q Plots. To investigate the strength of association with VO2peak, the relevant variables with significant associations in univariate analyses and cardiopulmonary and clinical variables known to affect VO2peak were selected for multiple regression analysis. Binary logistic regression analysis for assessing the odds ratios for achieving poor CRF (VO2peak <85% of expected as dependent variable) was performed, adjusting for preeclampsia and BMI in the first model. In the second regression model, the covariates PA level (steps per day) and hypertension were added and adjusted for in the analysis. The Wald test was used to determine the statistical significance of each independent variable. A p-value ≤0.05 was considered statistically significant for all analyses.
Results
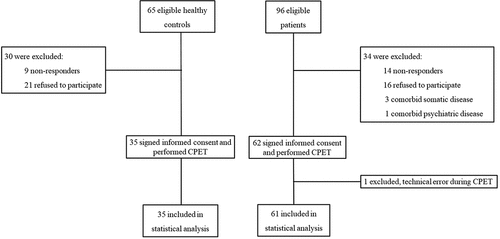
A total of 96 patients and 65 controls were identified and invited by an open invitation letter. Subsequently, 62 patients with previous severe preeclampsia and 35 healthy controls were included (). Twenty-eight (45%) patients had early-onset preeclampsia, and 34 (54%) had late onset.
The clinical characteristics of the study groups are presented in . Mean age (40 ± 3 years) and median time since delivery (7 ± 2 years) were similar across the study groups.
Table 1. Clinical characteristics of the study population.
Nine (13%) women in the early group and six (9%) women in the late group had suffered more than one preeclamptic pregnancy at follow-up. Six (9%) patients in the early group and four (6%) patients in the late group had one preeclamptic pregnancy before the index pregnancy with preeclampsia, which was subject to our retrospective clinical registrations. Two (3%) patients in the early group and one (1%) in the late group suffered preeclampsia in later pregnancies until follow-up. Time since delivery in the index pregnancy to follow-up was 7 ± 1 years in the study population and was comparable across the study groups (p = 0.25).
Two patients (3%) suffered from previous myocardial infarction after preeclampsia. The BMI was 15% higher in the preeclamptic groups (p < 0.01). Twenty-three patients (38%) were overweight, and 10 (17%) were obese. All controls were within the normal weight range. Hypertension was present in 18 patients (30%) before inclusion, and five (8%) patients were diagnosed with hypertension at inclusion. Hypertensive patients at follow-up were treated with beta-blocker (n = 9), AT-II or ACE-inhibitors (n = 9), calcium-inhibitors (n = 4) and diuretics (n = 1). In the hypertensive patients, seven (11%) patients had moderate hypertension and one patient had severe hypertension on repeated BP measurements at follow-up. LV systolic function was reduced, demonstrated by 14% lower EF (p < 0.01) and 19% lower GLS (p = 0.01) in the preeclamptic group compared with controls. Seven patients (11%) had an EF of 45% to 50%. Total cholesterol was normal in the preeclamptic groups (4.8 ± 0.8 mmol∙L−1; local laboratory reference value [3.9–7.8 mmol∙L−1]).
Cardiopulmonary exercise testing
All achieved the end criteria of RER ≥ 1.10, had high blood lactate levels (mean 10.8 ± 2.1 mmol∙L–1 for all participants), and reported a subjective maximal exertion rating of 17 on the 20-point Borg scale.
The difference in CRF measured during CPET between patients and controls is presented in . Both absolute (mL·min−1) and relative (mL·kg−1·min−1) VO2peak were 11 and 20% lower in the patients compared with the controls (p < 0.01), respectively. Correspondingly, COpeak values were 16% lower (p < 0.01). Relative VO2peak was poor in 23 (38%) patients, compared with two (6%) controls (p < 0.01). Of these patients, 12 had cardiac limitation during CPET, and 11 (18%) were classified as deconditioned or unfit without cardiopulmonary limiting factors. When compared with predictive reference values (Citation20), the difference in VO2peak for our patient group was 9% lower.
Table 2. Cardiopulmonary response during CPET.
No patients had signs of ischemia, exercise-induced hypoxemia, or abnormal pulmonary perfusion, demonstrated by normal saturation during maximal effort and a normal VE/VCO2 slope. In addition, there was no difference in VO2peak between the early and late preeclampsia groups.
The PA values are presented in . The patients spent 38% less time in MVPA levels and 14% more time being sedentary (p < 0.01) compared to controls. Twenty-one (45%) patients did not meet the WHO PA recommendations and were classified as physically inactive compared with four (17%) women in the control group (p = 0.02). The patients who fulfilled the WHO level of PA had a 16% higher VO2peak (p = 0.04) compared with those who did not.
Table 3. Physical activity in women at follow-up 7 years after severe preeclampsia.
In the 33 patients (54%) who were classed as overweight (n = 23) or obese (n = 10), hypertension and diabetes were present in 75% and 88% of them, respectively. In addition, their VO2peak was lower than in the lean patients (p = 0.04), and they spent less time in moderate to vigorous PA (p = 0.03).
The BP values at peak exercise were higher in the obese compared with lean patients (p = 0.04), and the prevalence of hypertensive response to PA was higher among adipose patients (60%) compared with lean (40%) patients.
Associations with cardiorespiratory fitness
Multivariable analyses for associations with VO2peak are presented in . Univariate analysis showed that BMI, SBPrest, DBPrest, SBPpeak, DBPpeak, HRpeak, COpeak, PA (counts per minute), and the time spent being sedentary were significantly associated with VO2peak. The final multivariable regression model showed that BMI, followed by PA (counts per minute), COpeak and DBPrest together accounted for 76% of the variability in the VO2peak.
Table 4. Multiple regression analysis using VO2peak as a dependent variable in women after severe preeclampsia.
A binomial logistic regression was conducted to investigate the effects of PE adjusted for BMI on the predictor variable VO2peak. This logistic regression model was statistically significant, with chi2 = 24.95, p < 0.001. The model explained 35.0% (Nagelkerke R2) of the variance in the patients with low CRF, correctly classifying 79% of cases. The PE groups were 20.3 times more likely to have poor CRF than the healthy controls. BMI was not a significant covariable to this model (p = 0.125).
Adding PA level added strength to the second regression model by including the continuous covariate steps per day, explained 58% of the variance in patients with low CRF, and correctly classified 81% of cases. This regression model showed that PE and lower PA levels increased the likelihood of having poor CRF.
Discussion
This cross-sectional study characterized CRF and PA levels in women 7 years after severe preeclampsia and showed significantly lower CRF and PA compared with healthy controls. About half of the patients with poor CRF were characterized as unfit with no evidence of cardiopulmonary limitations. In contrast, the other half with poor CRF had significant and clinical cardiac limitations shown by lower LV systolic and diastolic function at rest, lower COmax, and lower oxygen pulse at maximal effort. In addition, one-third of the patients showed a hypertensive response to exercise. No patients had clinical signs of heart failure. The strongest predictor for the reduction in VO2peak was BMI, followed by PA (counts per minute), COpeak and DBPrest. Preeclampsia and lower PA level increased the likelihood of having poor CRF.
CRF is stated to be one of the most important correlates for overall health status, where VO2peak provides the “gold standard” measurement of CRF (Citation11). The VO2peak reflects an individual’s CRF and is inversely associated with death from CVD or all-cause mortality in healthy as well as in unhealthy individuals (Citation10). Compared with healthy controls, the VO2peak was 20% lower (7.7 mL·kg−1·min−1) in a preeclamptic population, representing almost three decades of physiological aging (Citation20). In addition, the ventilatory threshold was correspondingly lower (23%) in the preeclamptic population, indicating an earlier anaerobic metabolism caused by lower oxygen availability and a lower CRF during sub-maximal exercise. From a physiological point of view, this lower VO2peak is unfortunate for these individuals because there is evidence that a decline in VO2peak of 1 metabolic equivalent of task (MET; 3.5 mL·kg−1·min−1) is associated with a high risk of developing lifestyle diseases (Citation36). Therefore, an effort to reduce the loss of CRF by increasing the PA level after pregnancy will be of significant importance.
To our knowledge, only one study has characterized CRF at long-term follow-up after preeclampsia. A nationwide Danish study measured VO2peak in 28 women with previous preeclampsia and 27 controls 8 years after giving birth (Citation12). Surprisingly, the VO2peak averages in the Danish preeclamptic population and their controls were as much as 38% and 16% higher, compared with the present patients and controls, respectively. This is despite the Danish patients performing CPET on an exercise bicycle, with a significantly lower HRpeak in both groups compared with HRpeak measured in the present study (167 and 176 vs 177 and 188 beats/min, respectively), indicating lower stress to the cardiopulmonary system. The significant negative gap in VO2peak in our study population compared with those in the Danish study is striking because it is well-known that steep treadmill walking generates more muscle mass activation, giving 10% to 20% higher VO2peak values compared with cycling (Citation37,Citation38). Nevertheless, one explanation for the difference in VO2peak might be the significantly higher BMI, higher occurrence of persistent hypertension after preeclampsia, and poorer LV systolic and diastolic function in our patient population. In addition, the patients included in our study had suffered from severe preeclampsia, whereas there were no data on the severity of preeclampsia in the Danish population. Also, milder preeclamptic disease is associated with a lesser risk of future CVD. These factors may have disadvantageous effect on CRF, which might explain some of the differences between the two studies.
One-third (28%) of the present patients were deconditioned or unfit, compared with 3% of the controls. This was demonstrated by normal echocardiographic indices at rest, normal ECG, normal gas exchange response during CPET, and no signs of ventilatory limitation or hypoxemia. Correspondingly, for the patients with cardiopulmonary limitations, the deconditioned patients also had a significantly higher prevalence of HRE, lower PA levels, and higher BMI values compared with the patients with a normal CRF. BMI, which was the most significant contributor to poor CRF in the preeclamptic group (), is a well-known contributor to lower VO2peak, lower PA level, and worse health (Citation35). Hence, the overweight and obese patients (60%) spent less time in moderate to vigorous PA and were significantly less physically active than the other patients. In addition, the obese patients had significantly higher diastolic BP at rest, and the prevalence of hypertensive response to PA was higher among adipose patients compared with the lean ones. Obesity can lead to hemodynamic alterations, neuro-hormonal and metabolic abnormalities, and LV remodeling and dysfunction (Citation39). Therefore, significant efforts to reduce weight gain after pregnancy in those with preeclampsia will also be of considerable importance.
Another key factor that might have contributed to poorer health was the SBP during CPET. One-third of the patients demonstrated HRE, defined as an SBP >196 mmHg during maximal effort (Citation33). With CRF in the lower ten percentile range (below 75% of predicted values), the incidence of HRE response to exercise was even higher. This is an important finding during CPET because HRE is an early warning signal with a prognostic value for developing hypertension later in life (Citation40). Data from the Framingham Offspring Cohort Study have shown that HRE has associations with traditional CVD risk factors (Citation41). They found a higher prevalence of increased LVM in individuals with HRE, probably from increased arterial stiffness and impaired endothelial function (Citation42). Here, the individuals with HRE demonstrated a significantly higher LVM than those with a normal exercise response. This aligns with the findings of a 10% greater LVM in individuals with HRE in the Framingham study cited above.
To our knowledge, this is the first study to measure PA objectively after a long-term follow-up in women after severe preeclampsia. The accelerometer data showed a significantly lower PA level and more time spent sedentary in the patients compared with controls. Consequently, a higher VO2peak was associated with higher PA levels, and the patients meeting the WHO recommendations (Citation34) had a significantly higher VO2peak than those who did not. Notably, the patients with cardiac limitation at rest also showed lower PA levels.
An earlier study has demonstrated low PA levels one year after preeclampsia after
PA modifies cardiac remodeling over time with increased LV compliance and improved ventriculo-arterial coupling (Citation43). Also, higher levels of PA before and during early pregnancy have demonstrated a lower risk of preeclampsia and vice versa (Citation44). Taken together, our findings emphasize the importance of maintaining PA to maintain good cardiovascular function in women with a history of preeclampsia.
Strength and limitations
The strengths of this study are the relatively long follow-up interval since severe preeclamptic pregnancies and the comprehensive cardiopulmonary evaluations with methods that allowed us to identify organ-specific impairments, including a high-technology cardiopulmonary exercise test and measure of COpeak.
The major limitation of this study was the cross-sectional observational design, which did not permit us to define causal relationships. We did not screen the control before inclusion in the study regarding weight and BMI; therefore, the controls were not matched to the patients in this regard.
Anthropometric composition, especially with abdominal obesity, is known to influence CRF (Citation45). The patients included here had a significantly higher BMI than controls, which may confound the results. Preeclampsia and CVD share risk factors such as obesity and metabolic syndrome. Data suggest that obese pregnant women with metabolic abnormalities have a high incidence of preeclampsia. Metabolic disturbances can lead to insufficient placentation with vascular dysfunction and ischemia, causing systemic endothelial dysfunction in preeclampsia (Citation46). Unfortunately, we did not record anthropometric data apart from the BMI. Total body fat and lean body mass through a DEXA scan could have distinguished the body composition of the populations and explored the relationship to CRF to a more considerable degree.
In addition, the lack of preconception and peripartum data prevented any analysis of longitudinal data—however, our comprehensive physiological assessment allowed for a thorough analysis of factors associated with VO2peak and PA.
An a priori sample size calculation was not performed before the study was conducted; The prevalence of severe preeclampsia is relatively rare and is reported in up to about 12 on 1000 deliveries (Citation47). This limits the availability of study participants, even across 6 years. We invited every woman diagnosed with severe preeclampsia (n = 96) between 2005 and 2010 to participate. We managed to include 62 patients. We could not provide data on the excluded patients, including the non-responders, as we did not have approval from the Regional Committee for Medical and Health Research Ethics or the local institutional board at Oslo University Hospital to register data on patients not providing written informed consent. We cannot rule out that non-response bias has affected our results’ external validity and generalizability.
Conclusions
Our study showed poor CRF in a majority of patients during long-term follow-up after severe preeclampsia. Adiposity, lower PA level, and hypertensive response to exercise were associated with lower VO2peak among the deconditioned or unfit patients. CPET can identify individuals with cardiovascular limitations and reveal cardiovascular risk factors where PA might be especially beneficial to reduce the risk of future CVD. Therefore, our findings highlight the need for targeted clinical follow-up and PA interventions after severe preeclampsia. The causes of exercise intolerance after this pregnancy disorder are incompletely understood, and exercise-interventional studies and more longitudinal data on affected women are needed.
Article highlights
The study provides a detailed insight into cardiorespiratory fitness and physical activity from long-term follow-up on women after preeclamptic pregnancies.
The strength of this study is the comprehensive cardiopulmonary evaluations with methods that allowed us to identify organ-specific impairments, including a high-technology cardiopulmonary exercise test and measure of cardiac output.
The findings highlight the need for targeted clinical follow-up and physical activity interventions after severe pre-eclampsia.
Contributorship statement
LG, EE, MEE, and EL designed the study. LG acquired the data. LG and EE analyzed the data. LG, MEE, and EE interpreted the data. LG and EE drafted the manuscript. All authors have critically reviewed the manuscript and approved the final version.
Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Ethics approval
The study was approved by the Norwegian Regional Committee for Medical and Health Research Ethics (REK Southeast, No. 2013-585b) and the local institutional board at Oslo University Hospital. Written informed consent was obtained from all study participants following the Declaration of Helsinki (Citation13).
Acknowledgements
The authors would like to thank all the study , Karine Udahl and physiologist Silje Rustad for technical assistance during the cardiopulmonary exercise testing. Mari Bratteteig contributed to the analyses of the accelerometer data.
Disclosure statement
The authors declare no conflicts of interest.
Data availability statement
No additional data is available.
Additional information
Funding
References
- Magnussen EB, Vatten LJ, Lund-Nilsen TI, et al. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335(7627):978. doi: 10.1136/bmj.39366.416817.BE
- Ainsworth BE, Haskell WL, Herrmann SD, et al. Compendium of physical activities: a second update of codes and MET values. Med & Sci In Sports & Ex. 2011 [2011];43(8):1575–10. doi: 10.1249/MSS.0b013e31821ece12
- Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56(1):166–171. doi: 10.1161/HYPERTENSIONAHA.110.150078
- Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66(8):497–506. doi: 10.1097/OGX.0b013e3182331028
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930. doi: 10.1016/j.ahj.2008.06.042
- Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135(6):e237–e60. doi: 10.1097/AOG.0000000000003891
- Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502. doi:10.1136/bmj.m3502
- den Ruijter H, Pasterkamp G, Rutten FH, et al. Heart failure with preserved ejection fraction in women: the dutch queen of hearts program. Neth Heart J. 2015;23(2):89–93. doi: 10.1007/s12471-014-0613-1
- Gronningsaeter L, Skulstad H, Quattrone A, et al. Reduced left ventricular function and sustained hypertension in women seven years after severe preeclampsia. Scand Cardiovasc J. 2022;56(1):292–301. doi: 10.1080/14017431.2022.2099012
- Guazzi M, Adams V, Conraads V, et al. EACPR/AHA joint scientific statement. clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012;33(23):2917–2927. doi: 10.1093/eurheartj/ehs221
- Force ERST, Palange P, Ward SA, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29(1):185–209. doi: 10.1183/09031936.00046906
- Ersbøll AS, Bojer AS, Hauge MG, et al. Long-term cardiac function after peripartum cardiomyopathy and preeclampsia: A Danish nationwide, clinical follow-up study using maximal exercise testing and cardiac magnetic resonance imaging. J Am Heart Assoc. 2018;7(20):e008991. doi: 10.1161/JAHA.118.008991
- World Medical A. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053
- Tranquilli AL, Brown MA, Zeeman GG, et al. The definition of severe and early-onset preeclampsia. statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013;3(1):44–47. doi: 10.1016/j.preghy.2012.11.001
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014
- Roman MJ, Okin PM, Kizer JR, et al. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the strong heart study. J Hypertens. 2010;28(2):384–388. doi: 10.1097/HJH.0b013e328333d228
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360. doi: 10.1093/ehjci/jew082
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805
- Graham BL, Brusasco V, Burgos F, et al. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017 [2017];49(1):1600016. doi: 10.1183/13993003.00016-2016
- Edvardsen E, Hansen BH, Holme IM, et al. Reference values for cardiorespiratory response and fitness on the treadmill in a 20- to 85-year-old population. Chest. 2013;144(1):241–248. doi: 10.1378/chest.12-1458
- Myatt L, Clifton RG, Roberts JM, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119(6):1234–1242. doi: 10.1097/AOG.0b013e3182571669
- Borg GA, Noble BJ. Perceived exertion. Exerc Sport Sci Rev. 1974;2(1):131–153. doi: 10.1249/00003677-197400020-00006
- Edvardsen E, Hem E, Anderssen SA, et al. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. Plos One. 2014;9(1):e85276. doi: 10.1371/journal.pone.0085276
- Charloux A, Lonsdorfer-Wolf E, Richard R, et al. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” fick method. Eur J Appl Physiol. 2000;82(4):313–320. doi: 10.1007/s004210000226
- Siebenmann C, Rasmussen P, Sorensen H, et al. Cardiac output during exercise: a comparison of four methods. Scand J Med Sci Sports. 2015;25(1):e20–7. doi: 10.1111/sms.12201
- Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exercise. 2005;37(11 Suppl):S531–43. doi: 10.1249/01.mss.0000185657.86065.98
- WHO. Obesity: preventing and managing the global epidemic. report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253.
- Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global lung function initiative reference values for the carbon monoxide transfer factor for caucasians. Eur Respir J. 2017;50(3):1700010. doi: 10.1183/13993003.00010-2017
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312
- American Thoracic S, American College of Chest P. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211
- HJ WK, Sue D, Sietsma K, et al. Principles of exercise testing and interpretation. 5 ed. Philadelphia:USA: Lippincott Williams & Wilkins; 2012.
- Poggio R, Arazi HC, Giorgi M, et al. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: a meta-analysis of the published literature. Am Heart J. 2010;160(6):1004–1014. doi: 10.1016/j.ahj.2010.08.037
- Sabbahi A, Arena R, Kaminsky LA, et al. Peak blood pressure responses during maximum cardiopulmonary exercise testing: Reference standards from FRIEND (Fitness Registry and the Importance of Exercise: A National Database). Hypertension. 2018;71(2):229–236. doi: 10.1161/HYPERTENSIONAHA.117.10116
- World Health Organization (2011) Global strategy on diet, Physical Activity And Health 2011 [ Last read 11th Feb 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/physical-activity.
- Hemmingsson E, Vaisanen D, Andersson G, et al. Combinations of BMI and cardiorespiratory fitness categories: trends between 1995 and 2020 and associations with CVD incidence and mortality and all-cause mortality in 471 216 adults. Eur J Prev Cardiol. 2022;29(6):959–967. doi: 10.1093/eurjpc/zwab169
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the st James women take heart project. Circulation. 2003;108(13):1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9
- Millet GP, Vleck VE, Bentley DJ. Physiological differences between cycling and running: lessons from triathletes. Sports Med. 2009;39(3):179–206. doi: 10.2165/00007256-200939030-00002
- Miyamura M, Honda Y. Oxygen intake and cardiac output during maximal treadmill and bicycle exercise. J Appl Physiol. 1972;32(2):185–188. doi: 10.1152/jappl.1972.32.2.-b185
- Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010;3(3):266–274. doi: 10.1016/j.jcmg.2009.10.012
- Schultz MG, La Gerche A, Sharman JE. Blood pressure response to exercise and cardiovascular disease. Curr Hypertens Rep. 2017;19(11):89. doi: 10.1007/s11906-017-0787-1
- Thanassoulis G, Lyass A, Benjamin EJ, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham heart study. Circulation. 2012;125(23):2836–2843. doi: 10.1161/CIRCULATIONAHA.111.063933
- Lauer MS, Levy D, Anderson KM, et al. Is there a relationship between exercise systolic blood pressure response and left ventricular mass? The Framingham heart study. Ann Intern Med. 1992;116(3):203–210. doi: 10.7326/0003-4819-116-3-203
- Hieda M, Howden E, Shibata S, et al. Impact of lifelong exercise training dose on ventricular-arterial coupling. Circulation. 2018;138(23):2638–2647. doi: 10.1161/CIRCULATIONAHA.118.035116
- Aune D, Saugstad OD, Henriksen T, et al. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25(3):331–343. doi: 10.1097/EDE.0000000000000036
- Farrell SW, Barlow CE, Willis BL, et al. Cardiorespiratory fitness, different measures of adiposity, and cardiovascular disease mortality risk in women. J Womens Health (Larchmt). 2020;29(3):319–326. doi: 10.1089/jwh.2019.7793
- Phipps EA, Thadhani R, Benzing T, et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–289. doi: 10.1038/s41581-019-0119-6
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306. doi: 10.1097/AOG.0b013e3181a45b25