ABSTRACT
This review pooled data from the literature to examine the association between preeclampsia (PE) and subsequent risk of breast cancer in women. Cohort studies published in the databases of PubMed, Embase, Scopus, and Web of Science up to 18 July 2023 were searched. Adjusted data were pooled to obtain the risk ratio (RR). Eleven studies with 15 cohorts and a cumulative sample size of 7,838,693 women were included. Meta-analysis of all studies demonstrated a reduced risk of breast cancer in women with PE as compared to those without PE (RR: 0.89 95% CI: 0.83, 0.95 p < 0.001 I2 = 50%). Follow-up ranged from 8 to 29.2 years. Results did not change during sensitivity analysis. Outcomes varied on subgroup analysis based on location, study type, data extraction method, incidence of breast cancer, and follow-up. To conclude, women with PE may have a reduced risk of breast cancer later in life. However, the risk reduction is minimal and may not have much clinical significance. The evidence is also limited by high inter-study heterogeneity and lack of adjustment of all possible confounders.
Introduction
Preeclampsia (PE) is a pregnancy-related multisystem syndrome seen in about 3–5% of all pregnancies (Citation1). It acts as a precursor to eclampsia and is characterized by hypertension and proteinuria occurring after 20 weeks of gestation (Citation1). The disorder is often associated with a spectrum of complications which include placental abruption, eclampsia, stroke, renal failure, fetal death, and preterm delivery (Citation2). While the pathophysiology of PE is unclear, its features are thought to originate from microangiopathy in various target organs. Reduced placental perfusion causes a release of anti-angiogenic factors in the circulatory system causing alteration of maternal systemic endothelial function (Citation3). This is hypothesized that altered endothelial function also leads to an increased risk of hypertension and other cardiovascular disorders later in life (Citation4).
In addition to cardiovascular disorders, PE has also been associated with a risk of future malignancies (Citation5–8). Specifically, the risk of estrogen-dependent breast cancer amongst PE women has been investigated by several studies. It has been suggested that PE leads to alteration of hormonal levels and hence could affect breast cancer risk and even other gynecological malignancies (Citation9). Calderon-Margalit et al. (2009) have shown that women diagnosed with PE have a 37% increased risk of breast cancer and a two-fold risk of ovarian cancer (Citation5). Another study by Walfisch et al. (2015) has reported that PE does not influence the risk of breast cancer or genital tract malignancies (Citation6). Contrastingly, recent studies have shown that PE may reduce the risk of breast cancer in the future (Citation7,Citation8).
Such discordant results amongst studies could be due to several reasons like variation in included participants, methods of identification of PE, adjusted confounders, variable follow-up, etc. In order to generate high-quality evidence, there is a need for a pooled analysis of long-term cohort studies reporting adjusted values of the association between PE and breast cancer. Previously, Sun et al. (2018) published their meta-analysis wherein they reported no association between PE and breast cancer (Citation10). However, their analysis could include just eight cohorts. With the publication of new evidence, there is a need for an updated review. Hence, the aim of the current study was to systematically examine published literature and conduct a pooled analysis to assess if PE influences the risk of future premenopausal or postmenopausal breast cancer in women.
Material and methods
Inclusion criteria
Protocol registration was done on PROSPERO and the review was allotted the number CRD42023442552. For inclusion in the review studies were to: 1. Be cohort in design (either retrospective or prospective) 2. Report the association between PE and subsequent risk of breast cancer. 3. Report the association using adjusted effect size. 4. Be published in the English language. There was no limitation on parity, sample size, and duration of follow-up.
Studies reporting cancer risk but not reporting specifically on breast cancer were excluded. Case–control studies, cross-sectional studies, duplicate studies, and those not reporting adjusted outcomes were also not eligible. In case articles reported overlapping data, the study with the maximum number of participants was eligible.
Search source and strategy
Studies for the review were identified by a literature search conducted on PubMed, Embase, Scopus, and Web of Science. Two reviewers were involved and the search included all articles available online from inception to 18 July 2023. To include gray literature, a separate search was conducted on Google Scholar. Also, the bibliography of the final included studies were hand-searched for any missed articles.
Keywords used were “pregnancy,” “complications,” “pregnancy hypertension,” “gestational hypertension,” “preeclampsia,” “eclampsia,” “breast,” AND “breast cancer.” Different search queries were formulated using “AND” and “OR” (Supplementary Table S1). These were also replicated across the different databases.
Two investigators separately examined the titles and abstracts of searched studies after electronic deduplication. Studies relevant to the review were identified while non-relevant articles were excluded. Selected studies underwent full-text analysis against the inclusion criteria. All discords between reviewers were solved by discussion.
Extracted data and risk of bias analysis
Two reviewers independently extracted relevant information from the studies which included: the name of the first author, publication year, region and database of the study, sample size, number of PE and breast cancer patients, identification of PE and breast cancer, parity of included women, pre- or post-menopausal breast cancer, adjusted covariates, follow-up, and effect size. Study details were then cross-matched and any discrepancies were resolved in discussion with the third author.
Two reviewers assessed the methodological quality of the observational studies by the Newcastle Ottawa Scale (NOS) (Citation11). Points were awarded for representativeness of the study cohort, comparability of groups, and measurement of outcomes.
Statistical analysis
PRIMA reporting guidelines were followed (Citation12). The meta-analysis was done on “Review Manager” (RevMan, version 5.3). Effect size data were extracted and entered into the software to derive pooled risk ratio (RR) with 95% confidence intervals (CI) of the association. Results were presented in the form of a forest plot. A random-effects model was preferred owing to methodological differences among the studies. Outliners were assessed using a sensitivity analysis involving the removal of one study at a time. Data was then presented in tabular format. Subgroup analysis was done based on the location of the study, study type, data extraction method, incidence of breast cancer, and follow-up. Publication bias was checked with funnel plots. The chi-square-based Q statistics and I2 statistic was used for inter-study heterogeneity. A p-value of <0.10 for Q statistic and I2 >50% meant substantial heterogeneity.
Results
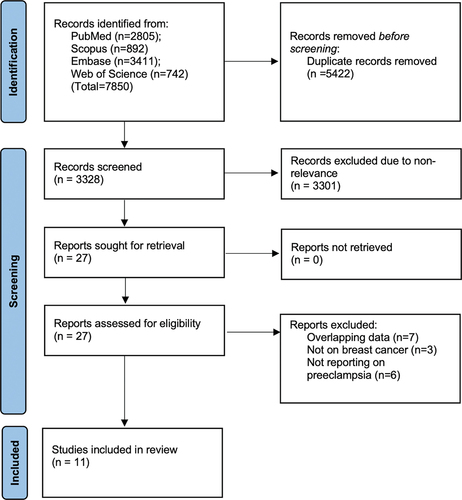
The entire literature search revealed 7850 articles (). After the removal of duplicates, 3328 studies remained. These underwent screening by the study investigators and 27 were chosen for complete text analysis. Based on the eligibility criteria, 11 were selected for inclusion (Citation5–8,Citation13–19).
The extracted data from the studies are presented in . Publication dates ranged from 2001 to 2023. Majority were Western studies conducted in populations of the USA, the UK, Sweden, Norway, Denmark, France, and Israel. The 11 studies included a total of 15 cohorts with a cumulative sample size of 7,838,693 women. Four studies were prospective while the rest were retrospective. The percentage of PE varied from 1.1% to 8.3% while the proportion of breast cancer cases ranged from 0.001% to 5.4%. The studies used medical records/international classification of disease codes or questionnaires to identify PE and breast cancer patients in the cohorts. Three studies included only primiparous females. Most studies did not report whether they focused only on pre- or postmenopausal breast cancer. The study of Nichols et al. (Citation7) assessed only premenopausal breast cancer while the study of Ma et al. (Citation15) examined the risk of only postmenopausal breast cancer. Three studies reported risk of both pre and postmenopausal breast cancer. The adjusted confounders differed across studies while the follow-up ranged from 8 to 29.2 years. Based on the NOS scale the studies were given a score of 7 to 9.
Table 1. Details of included studies.
PE, preeclampsia; NR, not reported; NOS, Newcastle Ottawa scale; P, prospective; R, retrospective; ICD, international classification of diseases
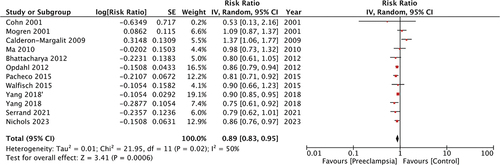
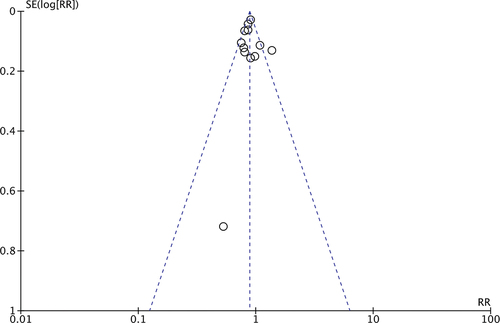
Meta-analysis of all studies with 15 cohorts demonstrated a reduced risk of breast cancer in women with PE as compared to those without PE (RR: 0.89 95% CI: 0.83, 0.95 p < 0.001 I2 = 50%) (). We did not note any publication bias on the funnel plot (). The results were consistent with the sequential exclusion of individual studies. The sensitivity analysis did not find any change in the significance of the results ().
Table 2. Details of sensitivity analysis.
The results of the subgroup analysis are shown in . Based on location, the results showed a reduced risk of breast cancer with PE in European studies but not in studies from the USA or Israel. For retrospective studies, the results demonstrated a tendency of reduced risk of breast cancer but the upper end of the 95% CI was 1. The results also turned non-significant for studies including only primiparous women, with breast cancer incidence of ≥2% and follow-up ≥20 years.
Table 3. Subgroup analyses.
Discussion
The association between PE and the risk of cancer is indeed intriguing and has been a subject of prior meta-analyses. Wang et al. (Citation20) in the pooled analysis have shown that PE results in an 82% increased risk of ovarian cancer but has no impact on the risk of uterine cancers. Jardao et al. (Citation21) in a recent meta-analysis of seven studies have shown no influence of PE on the subsequent risk of endometrial cancer. Sun et al. (Citation10) combined data from eight cohorts to demonstrate no association between PE and subsequent risk of breast cancer (RR: 0.93 95% CI: 0.82, 1.06 I2 = 61%). In this study, we conducted an updated literature search to include three more studies with seven cohorts to substantially increase the statistical power of the analysis as compared to the previous review (Citation10). But by pooling data from 11 studies with 15 cohorts including around 7.8 million women, this meta-analysis noted a statistically significant 11% reduced risk of breast cancer amongst women with a history of preeclampsia. The robustness of the results was validated during the sensitivity analysis wherein the risk remained significantly reduced on the exclusion of any included study.
Importantly, there could be several confounders that could influence the risk of breast cancer in women with PE. To partly overcome such limitations, only studies reporting adjusted data were included in the meta-analysis. Nevertheless, there was much variation in the confounders adjusted and this could have been an important source of the substantial inter-study heterogeneity noted in the review. Furthermore, variations in the study populations, location, data extraction method, and follow-up could have contributed to the heterogeneity. To examine the effect of such variations, multiple subgroup analyses were conducted and the results turned non-significant for studies based in the USA and Israel, those including only primiparous women, with breast cancer incidence of ≥2% and follow-up ≥20 years. This could possibly be due to the minimal risk reduction of just 11% noted in the meta-analysis with the 95% CI ranging from 5% to 17%. Such minimal protective effect dissipated in multiple subgroup analyses owing to a limited number of studies.
The pathophysiological mechanism behind the reduced risk of breast cancer with PE is not very clear, however, several mechanisms have been proposed. PE has been associated with an elevated inflammatory response involving T-helper cell (Th1)-like inflammatory reaction at the cost of Th2 response (Citation22). Also, placental hypoxia possibly due to the inflammatory reaction leads to excessive production of vascular endothelial growth factor (VEGF) inhibitors like soluble vascular endothelial growth factor receptor-1 (sFlt1) which deactivates VEGF causing the advent of PE (Citation23,Citation24). There is subsequent underdevelopment of vascularity of the placenta which releases antiangiogenic factors in the maternal circulation (Citation3). Placental hypoxia is also associated with oxidative stress and trophoblast immaturity (Citation25,Citation26). Research shows that a positive association exists between second- to third-trimester blood pressure increase and circulating levels of antiangiogenic factors in the mother (Citation27). Importantly, Th1-like inflammatory reaction and antiangiogenic mechanisms are shown to improve prognosis in breast cancer. Kristensen et al. (Citation28) have demonstrated that breast cancer cases acquiring a gene signature that supports a Th1-based immune response compared to a Th2-driven humoral response have a better prognosis. Angiogenic factors like VEGF and placental growth factor have been found in increased quantities in cancerous tissue and are associated with poor shorter recurrence-free survival and increased mortality in breast cancer patients (Citation29,Citation30). This has prompted the use of anti-angiogenic therapies like bevacizumab for the treatment of breast cancer (Citation31). The antiangiogenic profile seen with PE declines rapidly after pregnancy (Citation32), however, there may be persistent differences that could contribute to the lower risk of breast cancer with PE in the long term. Another possible explanation is the reduced mammographic density noted in women with preeclampsia. Yang et al. (Citation8) have shown that PE patients and their sisters have reduced mammographic density which is widely considered an immediate phenotype for breast cancer. Genetic factors shared between preeclampsia and breast development could therefore alter the risk of breast cancer.
Studies have reported that the association between PE and breast cancer could be confounded by preterm birth. Nichols et al. (Citation7) have shown that reduce risk of breast cancer is noted only amongst women with hypertensive disorders delivering at term but not amongst those with preterm deliveries. Opdahl et al. (Citation17) have also shown that pregnancy duration is inversely associated with breast cancer risk in women with PE with longer duration reducing the chances of breast cancer. It has been postulated that early presentation of PE is associated with a stronger antiangiogenic profile as compared to late-onset PE which could be due to metabolic diseases like obesity and hence with reduced protective effect (Citation7).
The strength of the review includes the updated literature search and inclusion of new large sample size studies thereby presenting the most updated and comprehensive evidence on the subject. Only cohort studies were included and case-control and cross-sectional studies were excluded to gather the best possible evidence. Multiple subgroup analyses and sensitivity analyses were carried out to examine the results. Our findings must be interpreted with the following limitations. Despite limiting the meta-analysis to only longitudinal cohort studies, the current review at best presents a possibility of reduced risk of breast cancer with PE and does not demonstrate a causative relationship. The inherent bias in observational studies like errors in data collection, record-keeping, and loss of follow-up could have affected the results. Also, we could not differentiate between the risk of pre- and post-menopausal breast cancer due to paucity of data. Secondly, the scarcity of data on confounders in different cohorts meant that not all variables could be adjusted. Several known and unknown confounders missed in the analysis could have potentially skewed the outcomes. For example, only one study (Citation19) adjusted for preexisting hypertension in the analysis. Hypertension has been demonstrated as an important risk factor for breast cancer especially in postmenopausal women (Citation33). Also, a recent study has shown that the molecule G protein coupled receptor kinase 4, a risk factor for hypertension, is present in breast cancer cells but not in normal breast cells (Citation34). Lack of inclusion of such important confounders is a significant drawback of the review. Thirdly, a few studies recorded PE using questionnaires which could be influenced by recall bias. Lastly, data were available from only limited Western studies and may not be representative of the global population.
Conclusions
Women with PE may have a reduced risk of breast cancer later in life. However, the risk reduction is minimal and may not have much clinical significance. The evidence is also limited by high inter-study heterogeneity and lack of adjustment of all possible confounders.
Article highlights
Current meta-analysis pooled data from 11 studies with 7.8 million participants.
Women with preeclampsia had 11% reduced risk of breast cancer.
Risk reduction was minimal and may not have much clinical significance.
Supplemental Material
Download MS Word (21.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2265482
Additional information
Funding
References
- Malik A, Jee B, Gupta SK. Preeclampsia: disease biology and burden, its management strategies with reference to India. Pregnancy Hypertens. 2019;15:23–8. doi: 10.1016/J.PREGHY.2018.10.011
- Bokslag A, van Weissenbruch M, Mol BW, et al. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. doi: 10.1016/J.EARLHUMDEV.2016.09.007
- Tomimatsu T, Mimura K, Matsuzaki S, et al. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci. 2019;20(17):4246. doi: 10.3390/IJMS20174246
- Paauw ND, Lely AT. Cardiovascular sequels during and after preeclampsia. Adv Exp Med Biol. 2018;1065:455–470. doi: 10.1007/978-3-319-77932-4_28
- Calderon-Margalit R, Friedlander Y, Yanetz R, et al. Preeclampsia and subsequent risk of cancer: update from the Jerusalem perinatal study. Am J Obstet Gynecol. 2009;200(1):0.e63.1–0.e63.5. doi: 10.1016/J.AJOG.2008.06.057
- Walfisch A, Kessous R, Davidson E, et al. Pre-eclampsia and future female malignancy. Hypertens Pregnancy. 2015;34(4):456–463. doi: 10.3109/10641955.2015.1071838
- Nichols HB, House MG, Yarosh R, et al. Hypertensive conditions of pregnancy, preterm birth, and premenopausal breast cancer risk: a premenopausal breast cancer collaborative group analysis. Breast Cancer Res Treat. 2023;199(2):323–334. doi: 10.1007/S10549-023-06903-5
- Yang H, He W, Eriksson M, et al. Inherited factors contribute to an inverse association between preeclampsia and breast cancer. Breast Cancer Res. 2018;20(1). doi: 10.1186/S13058-017-0930-6
- Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertens (Dallas, Tex 1979). 2013;61(2):480–487. doi: 10.1161/HYPERTENSIONAHA.111.201624
- Sun M, Fan Y, Hou Y, et al. Preeclampsia and maternal risk of breast cancer: a meta-analysis of cohort studies. J Matern Fetal Neonatal Med. 2018;31(18):2484–2491. doi: 10.1080/14767058.2017.1342806
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 2020 October 30. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906
- Mogren I. Long-term impact of reproductive factors on the risk of cervical, endometrial, ovarian and breast cancer. Acta Oncol. 2001;40(7):849–854. doi: 10.1080/02841860152703481
- Cohn BA, Cirillo PM, Christianson RE, et al. Placental characteristics and reduced risk of maternal breast cancer. J Natl Cancer Inst. 2001;93:1133–1140. doi: 10.1093/JNCI/93.15.1133
- Ma H, Henderson KD, Sullivan-Halley J, et al. Pregnancy-related factors and the risk of breast carcinoma in situ and invasive breast cancer among postmenopausal women in the California teachers study cohort. Breast Cancer Res. 2010;12(3). doi: 10.1186/BCR2589
- Bhattacharya S, Prescott GJ, Iversen L, et al. Hypertensive disorders of pregnancy and future health and mortality: a record linkage study. Pregnancy Hypertens. 2012;2(1):1–7. doi: 10.1016/J.PREGHY.2011.08.116
- Opdahl S, Romundstad PR, Alsaker MDK, et al. Hypertensive diseases in pregnancy and breast cancer risk. Br J Cancer. 2012;107(1):176–182. doi: 10.1038/BJC.2012.195
- Pacheco NLP, Andersen AMN, Kamper-Jørgensen M. Preeclampsia and breast cancer: the influence of birth characteristics. Breast. 2015;24(5):613–617. doi: 10.1016/J.BREAST.2015.06.006
- Serrand C, Mura T, Fabbro-Peray P, et al. Assessment of all-cause cancer incidence among individuals with preeclampsia or eclampsia during first pregnancy. JAMA Netw Open. 2021;4(6):e2114486. doi: 10.1001/JAMANETWORKOPEN.2021.14486
- Wang F, Zhang W, Cheng W, et al. Preeclampsia and cancer risk in women in later life: a systematic review and meta-analysis of cohort studies. Menopause. 2021;28(9):1070–1078. doi: 10.1097/GME.0000000000001806
- Jordao H, Herink K, Ka E, et al. Pre-eclampsia during pregnancy and risk of endometrial cancer: a systematic review and meta-analysis. BMC Womens Health. 2023;23: doi: 10.1186/S12905-023-02408-X
- Boij R, Svensson J, Nilsson-Ekdahl K, et al. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol. 2012;68(3):258–270. doi: 10.1111/J.1600-0897.2012.01158.X
- Palm M, Basu S, Larsson A, et al. A longitudinal study of plasma levels of soluble fms-like tyrosine kinase 1 (sFlt1), placental growth factor (PlGF), sFlt1: PlGF ratio and vascular endothelial growth factor (VEGF-A) in normal pregnancy. Acta Obstet Gynecol Scand. 2011;90(11):1244–1251. doi: 10.1111/J.1600-0412.2011.01186.X
- Redman CWG, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):38–42. doi: 10.1016/J.PLACENTA.2008.11.021
- Fantone S, Mazzucchelli R, Giannubilo SR, et al. AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem Cell Biol. 2020;154(3):339–346. doi: 10.1007/S00418-020-01892-8
- Tossetta G, Fantone S, Piani F, et al. Modulation of NRF2/KEAP1 signaling in preeclampsia. Cells. 2023;12(11):1545. doi: 10.3390/CELLS12111545
- Troisi R, Braekke K, Harsem NK, et al. Blood pressure augmentation and maternal circulating concentrations of angiogenic factors at delivery in preeclamptic and uncomplicated pregnancies. Am J Obstet Gynecol. 2008;199(6):.e653.1–.e653.10. doi: 10.1016/J.AJOG.2008.06.030
- Kristensen VN, Vaske CJ, Ursini-Siegel J, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A. 2012;109(8):2802–2807. doi: 10.1073/PNAS.1108781108
- Maae E, Olsen DA, Steffensen KD, et al. Prognostic impact of placenta growth factor and vascular endothelial growth factor a in patients with breast cancer. Breast Cancer Res Treat. 2012;133(1):257–265. doi: 10.1007/S10549-012-1957-0
- Parr C, Watkins G, Boulton M, et al. Placenta growth factor is over-expressed and has prognostic value in human breast cancer. Eur J Cancer. 2005;41(18):2819–2827. doi: 10.1016/J.EJCA.2005.07.022
- Varella L, Abraham J, Kruse M. Revisiting the role of bevacizumab in the treatment of breast cancer. Semin Oncol. 2017;44(4):273–285. doi: 10.1053/J.SEMINONCOL.2017.10.010
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMOA031884
- Han H, Guo W, Shi W, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep. 2017;7(1). doi: 10.1038/SREP44877
- Yue W, Tran HT, Wang JP, et al. The hypertension related gene G-Protein coupled receptor kinase 4 contributes to breast cancer proliferation. Breast Cancer (Auckl). 2021;15:117822342110157. doi: 10.1177/11782234211015753