ABSTRACT
Background
HELLP syndrome, featuring hemolysis, elevated liver enzymes, and thrombocytopenia, is life-threatening disease of pregnancy that triggers comorbidities in both pregnant women and the fetus/newborn. This study provides an updated systematic review and meta-analysis of relevant studies to assess the therapeutic efficacy of corticosteroids in maternal and neonatal outcomes.
Methods
Randomized control trials (RCTs) regarding the use of corticosteroids in the HELLP population from three electronic databases, including Ovid MEDLINE, Ovid EMBASE, andCochrane Central Register of Controlled Trials, were searched from database inception to 23 March 202323 March 2023.
Results
A total of 485 patients treated with corticosteroids from 7 RCTs were included. Compared to placebo, corticosteroids therapy failed to significantly improve the maternal outcomes regard to maternal morbidity (RR = 1.36, 95%CI [0.45, 4.10]), eclampsia (RR = 1.16, 95%CI [0.76, 1.77]), acute renal failure (RR = 0.71, 95%CI [0.41, 1.22]), pulmonary edema (RR = 0.34, 95%CI [0.10, 1.15]) and oliguria (RR = 1.08, 95%CI [0.75, 1.54]). In addition, pooled data showed that it wasn’t significant differences between corticosteroids therapy and placebo regarding neonatal outcomes.
Conclusions
This study compared the efficacy of corticosteroids in patients with HELLP syndrome, revealing that corticosteroids did not provide any significant benefit in clinical outcomes for pregnant women and newborns with HELLP. The conclusions of this study must be verified by a larger sample of high-quality RCTs.
Introduction
HELLP syndrome, an acronym for a series of symptoms, is a rare pregnancy complication and a severe form of preeclampsia. It was first described in 1982 by Weinstein as a syndrome that consists of three characteristic conditions, hemolysis, elevated liver enzymes, and thrombocytopenia (H=Hemolysis, EL=Elevated Liver enzymes, LP=Low Platelets) (Citation1). The prevalence of HELLP syndrome is 0.5% to 0.9%, with the majority of cases occurring between 27 and 34 weeks of gestation, 70% of cases occurring in the prenatal period, and 11% within the third trimester of pregnancy (Citation2). HELLP syndrome is associated with a risk of multiple organ failure and even death (Citation3,Citation4), causing a wide range of nonspecific symptoms and complications, including disseminated intravascular coagulation (DIC), maternal mortality, eclampsia, acute renal failure, hemorrhagic manifestations, pulmonary edema, and oliguria in the mother and perinatal death, intrauterine growth restriction, preterm birth, neonatal thrombocytopenia, leukopenia, neutropenia, and respiratory distress syndrome in the fetus. However, neonatal morbidity and death are related to gestational age rather than the presence or absence of HELLP syndrome at gestational weeks 24 to 36 (Citation5). Although the etiology of this disease is still unknown, it could be related to invading fetal trophoblast injury caused by immune intolerance in the early stages of pregnancy. Furthermore, age, history of pregnancy, history of severe preeclampsia, and history of HELLP syndrome are predisposing factors of HELLP (Citation6). Patients usually first show symptoms of discomfort before 36 weeks of gestation, approximately half of the women report nausea and vomiting, and abdominal pain occurs in 90%. Moreover, others develop symptoms similar to those of a nonspecific viral syndrome. HELLP is often life-threatening and interferes with the safety of pregnant women and their fetuses if not addressed in time (Citation7). In addition, relevant evidence (Citation8) suggested that enhanced screening for preeclampsia will be beneficial to reduce the development of potential HELLP syndrome.
At present, there is no definitive cure for HELLP syndrome, and patients with the syndrome can rapidly deteriorate due to multiple system organ failure. To some extent, the best effort of the attending physician is to alleviate the patient’s symptoms. The mainstay of treatment is delivery and removal of the placenta. Other treatments have been developed and applied, including maternal administration of corticosteroids and therapeutic plasma exchange (Citation9). Relevant studies have already been conducted providing clinicians with multidisciplinary viewpoints of this syndrome and have greatly improved the understanding of the management and even the subsequent therapeutic treatment. Regarding treatment, Wallace“s” study was designed to illustrate the mechanisms of action of corticosteroids in women with HELLP syndrome. The study concluded that dexamethasone administration interferes with the release of both antiangiogenic and inflammatory factors that are suggested to play role in the pathophysiology of HELLP syndrome (Citation10). At the gene level, a whole exome sequencing performed in isolated familial cases of HELLP syndrome using large-scale genomic approaches to identify the genetic origin of HELLP found that five sequence variants generated premature stop codons in genes playing an essential role in placental physiology and six variants led to destabilization of protein structure as they had significant energy and residue interaction-related changes (Citation11). Studies have not identified any novel clinically useful molecular biomarkers capable of identifying patients with HELLP syndrome. One study was the stepping stone of the biomarker fields, and revealed the untiring effort of scientists to one day identify a suitable protein marker for HELLP syndrome (Citation12).
Several studies have been published in the literature on the use of corticosteroids in the treatment of HELLP syndrome, although it is not possible to distinguish a definitive outcome of this treatment (Citation7,Citation13–16). One of these studies reported significant improvements in maternal platelet function, the degree of hemolysis, and changes in liver transaminases levels following with corticosteroid treatment (Citation17). In another study, the use of corticosteroids was found to decrease the morbidity and mortality of the fetus reaching at less than 34 weeks of gestation. However, to date, there is no definitive evidence that corticosteroids contribute to an overall improvement in morbidity and mortality in pregnant women or to improvements in perinatal mortality (Citation16). Moreover, the benefits of corticosteroids in the treatment of HELLP syndrome patients are controversial in the literature. Therefore, we conducted this systematic review and meta-analysis with the aim of determining the effectiveness of corticosteroids in this life-threatening condition involving the mother and fetus, and to compare pathological phenomena emerging from randomized controlled trials (RCTs) to improve clinical decision-making.
Materials and methods
This systematic review and meta-analysis was conducted in accordance with the Guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Citation18).
Search strategy
A rigorous search strategy was implemented to include all eligible studies in Ovid MEDLINE, Ovid EMBASE, and the Cochrane Central Register of Controlled Trials, taking HELLP syndrome (hemolysis, elevated liver enzymes and low platelet count/levels), corticosteroids, dexamethasone, betamethasone as MeSH terms and keywords to searched for articles published from the inception of the database until 23 March 2023. The detailed search strategy was shown in Supplementary Method 1.
Eligibility criteria
The inclusion criteria were: (1) Population: pregnant women with HELLP syndrome; (2) Intervention: corticosteroids treatment as an intervention; (3) Comparison: patients treated with placebo; and (4) Outcome: outcomes were defined as maternal and neonatal outcomes. Maternal outcomes included changes in platelet count, accompanied by comorbidities in which DIC, maternal mortality, eclampsia, acute renal failure, hemorrhagic manifestations, pulmonary edema, and oliguria. Neonatal outcomes included fetal death (fetal death, placental abruption, intrauterine growth retarded, severe prematurity, severe respiratory distress syndrome (RDS) and extensive periventricular leukomalacia), birth weight, gestational age at delivery, mechanical ventilation, duration of admission to neonatal intensive care unit (NICU), the 5-min Apgar score (Citation15), RDS grade III – IV, intraventricular hemorrhage (IVH) grade III – IV, necrotizing enterocolitis grade 2–3, bronchopulmonary dysplasia, pulmonary hypertension, and severe bronchopulmonary dysplasia; and (5) study design: RCT.
The exclusion criteria were as follows: (1) mothers who did not present hemolysis, elevated liver enzymes, or low platelet counts as defined clinically or based on biochemical markers, both during pregnancy and after delivery, and in their babies; (2) data were incomplete and could not be extracted; and (3) studies with a unclear numerical values of valuable data.
Study selection and data extraction
The literature was screened independently by two authors. They also independently extracted the data using a standardized data extraction form and findings were cross-checked. Disagreements between the two authors were resolved by discussion. Each author independently evaluated each study and extracted basic information, including the author’s name, year and country of publication, maternal age, gestational age, sample size, delivery mode (vaginal/cesarean), duration of treatment, dosage, and usage of medication.
Quality assessment and risk of bias
The Cochrane risk of bias tool (Citation19) was used to assess the methodological quality of eligible trials, with quality assessment and risk of bias completed independently by two reviewers. This included randomization grouping and allocation concealment (selection bias), study subject and investigator blinding (implementation bias) and blinding of outcome measures (measurement bias), incomplete outcome data (attrition bias), and reporting of all results in the study protocol (reporting bias). Differences between reviewers’ opinions were resolved by discussion with the other authors.
Data synthesis and analysis
Risk ratios (RR) or standardized mean difference (SMD) (Citation20) with 95% confidence intervals (CI) were used for dichotomous and continuous data. The heterogeneity of the meta-analyses was measured by calculating I2. If I2 ≥40%, the random-effects model was employed, otherwise, the fixed model was adopted. When the number of included studies was no less than three, meta-analysis was used for pooling result. Otherwise, descriptive analysis of the results will be adopted. Additionally, random effect models and fixed effect models were used for the results with generic inverse variance weighting depending on statistical homogeneity, computing risk estimates with 95% CI, using RevMan 5.4 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Study selection
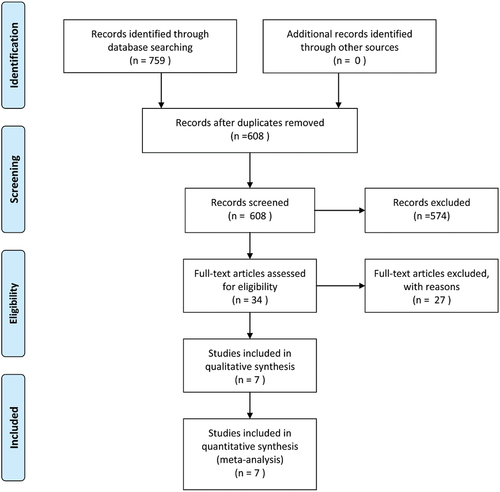
A total of 445 relevant articles were identified in the meta-analysis literature search. After selecting the title and abstract, 430 articles were excluded because they were inconsistent with eligibility criteria. After carefully reading the full text of each article, 3 more articles were also excluded. Finally, a total of seven studies (Citation14,Citation21–26) were eligible and included in the meta-analysis ().
Basic characteristics of the included studies
From the seven included studies, all of which were RCTs, a total of 485 patients treated with corticosteroids were recruited. shows the basic characteristics of the included studies in which the author, published year, author’s nation, type of trial, samples, method of delivery (vaginal or cesarean), maternal age, gestational age, dose and medication, and duration of hospitalization were included. With regard to the study origins, four studies were from the United States, one was from the Netherlands, and the remaining two studies were from Brazil and Turkey, respectively; all data were pooled for this meta-analysis. Furthermore, the data distribution revealed that in the category of maternal age, gestational age, delivery, and duration of hospitalization, the higher values represented patients who were treated with corticosteroids, while the values were patients with placebo-treated consequences. Among the RCTs, one study (Citation26) specifically defined the neonatal outcomes related to cause of death (fetal death, placental abruption, delayed intrauterine growth, severe prematurity, severe RDS and extensive periventricular leukomalacia), birth weight, and gestational age at delivery, Mechanical ventilation, duration of stay in the NICU, 5-min Apgar score, grade III-IV RDS, IVH grade III – IV, grade 2–3 necrotizing enterocolitis, bronchopulmonary dysplasia, pulmonary hypertension, and severe bronchopulmonary dysplasia in .
Table 1. Basic characteristics of including studies.
Quality assessment of the included studies
provides the risk of bias assessments. Five of the seven randomized controlled trials (RCTs) were judged to have a considerable risk of bias and the remaining two studies presented some limitations. Most concerns arose from the missing outcome data, where the variables following administration of corticosteroids, such as liver enzyme levels, were unavailable in the majority of the included studies. This meant that it was difficult to judge the effectiveness with which corticosteroids versus placebo groups viewed their interventions as equivalent.
Table 2. Risk of bias for randomized controlled trials.
Result of meta-analysis
Maternal outcomes
Changes in platelet count
Only Ozer’s study (Citation14), involving 60 pregnant women, reported fluctuation of platelet counts. Ozer’s study (Citation14) showed that platelet counts after the use of corticosteroids (81.2%, 95% CI [43%, 533%]) and placebo (94.5%, 95% CI [24%, 627%]) were increased, but there was no statistical difference (P = 0.23).
Disseminated intravascular coagulation
There were two studies (Citation14,Citation26) with a sample size of 91 reported DIC cases in the context of HELLP syndrome. None of the studies described the effectiveness of corticosteroid treatment. Ozer’s study (Citation14) showed that one DIC occurred in the corticosteroids-only group, but Pieter’s study (Citation26) showed that one DIC occurred in the placebo-only group.
Maternal mortality
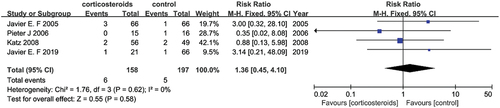
Four studies (Citation22–24,Citation26) with a sample size of 355 reported maternal mortality after corticosteroids therapy for the duration of the HELLP syndrome. None of these studies showed the effectiveness of corticosteroids. Furthermore, no significant differences were observed between the two groups (RR = 1.36, 95% CI [0.45, 4.10]) without significant heterogeneity (I2 = 0%) ().
Eclampsia
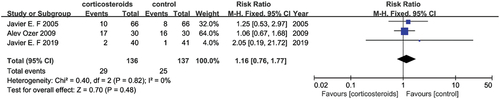
Three studies (Citation14,Citation22,Citation23) reported eclampsia as a feature of the HELLP syndrome, the sample size included 273 patients combined for the meta-analysis. None of the studies showed the effectiveness of corticosteroids. An effect was observed in the pooled result, which indicated there was no discrimination within corticosteroids therapy versus placebo (RR = 1.16, 95% CI [0.76, 1.77]) with no significant heterogeneity (I2 = 0%) ().
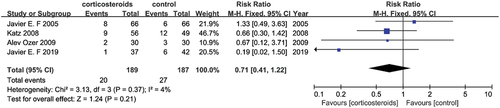
Acute renal failure
Four studies (Citation14,Citation22–24) with a sample size of 376 reported case control data, and the entire dataset could be incorporated into a meta-analysis. None of the studies reported on the effectiveness of treatment with corticosteroids. The pooled data did not show any correlation between intervention, corticosteroids versus placebo (RR = 0.71, 95% CI [0.41, 1.22]) with no significant heterogeneity (I2 = 4%) ().
Hemorrhagic manifestations
Two studies (Citation14,Citation24) with a sample size of 165 reported hemorrhagic manifestations during the HELLP syndrome. Ozer’s study (Citation14) showed that 6 hemorrhagic manifestations occurred in the corticosteroids group, and 13 hemorrhagic manifestations occurred in the placebo group, there was statistical difference (P = 0.01). However, Katz’s study (Citation24) showed that 20 hemorrhagic manifestations occurred in the corticosteroids group, and 16 hemorrhagic manifestations occurred in the placebo group, but there was not statistical difference (P = 0.74).
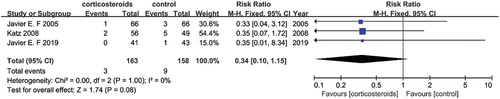
Pulmonary edema
The total sample size included 321 patients from three studies (Citation22–24). The effectiveness corticosteroids to treat lung edema caused by HELLP syndrome was analyzed. None of the studies showed the effectiveness of corticosteroids. Compared to the control, there was no association in the corticosteroids treated groups versus the placebo groups (RR = 0.34, 95% CI [0.10, 1.15]) of pulmonary edema, and there was no significant heterogeneity (I2 = 0%) in .
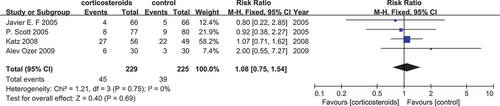
Oliguria
Four studies (Citation14,Citation21,Citation22,Citation24) with a sample size of 454 were combined in a meta-analysis to evaluate the phenomenon of oliguria following the administration of corticosteroids or placebo. Importantly, none of the studies showed the effectiveness of corticosteroids. The pooled data did not show any relationship with oliguria in the corticosteroids-treated groups (RR = 1.08, 95% CI [0.75, 1.54]). However, two studies (Citation21,Citation22), which represented the largest sample size revealed a plausible effectiveness of corticosteroids with the calculation of RR = 0.80, 95% CI [0.22, 2.58] and RR = 0.92, 95% CI [0.38, 2.27], respectively, together with a not significant heterogeneity (I2 = 0%) in .
Neonatal outcomes
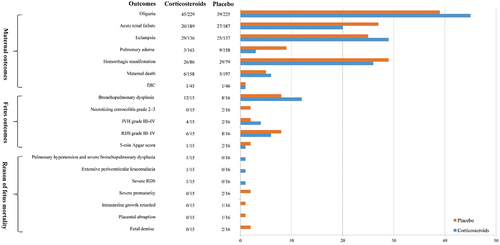
Only one study (Citation26) with a total sample size of 31 reported neonatal outcomes showed there were no significant differences between the corticosteroids and the placebo-treated group. Among all neonatal outcomes, four outcomes with continuous measures were illustrated: birth weight (MD = −32.00, 95% CI [−182.43, 118.43]), gestational age at delivery (MD = −0.40, 95% CI [−1.40, 0.60]), mechanical ventilation (MD = 0.80, 95% CI [−9.03, 10.63]), duration of admission to the NICU (MD = −3.80, 95% CI [−20.09, 12.49]), following with five dichotomous measures illustrated as: subsequent 5-min Apgar score (RR = 0.53, 95% CI [0.05, 5.29]), RDS grade III – IV (RR = 0.80, 95% CI [0.36, 1.76]), IVH grade III – IV (RR = 2.13, 95% CI [0.46, 9.99]), grade 2–3 necrotizing enterocolitis (RR = 0.21, 95% CI [0.01, 4.10]), or bronchopulmonary dysplasia (RR = 1.60, 95% CI [0.92, 2.78]). Besides, outcomes of fetus death reasons were also extracted explained as: total fetus deaths (RR = 0.53, 95% CI [0.16, 1.76]), fetal demise (RR = 0.21, 95% CI [0.01, 4.10]), placental abruption (RR = 0.35, 95% CI [0.02, 8.08]), delayed intrauterine growth (RR = 0.35, 95% CI [0.02, 8.08]), severe prematurity (RR = 0.21, 95% CI [0.01, 4.10]), RDS (RR = 3.19, 95% CI [0.14, 72.69]), extensive periventricular leukomalacia (RR = 3.19, 95% CI [0.14, 72.69]), pulmonary hypertension and severe bronchopulmonary dysplasia (RR = 3.19, 95% CI [0.14, 72.69]).
Discussion
This meta-analysis determined that there were no significant differences in outcomes between corticosteroid treatment of patients with HELLP syndrome, compared to controls. Moreover, this meta-analysis confirmed and updated the results of previous meta-analyses (Citation27). We examined the effects of corticosteroids on comorbidities, such as DIC, maternal mortality, eclampsia, acute renal failure, hemorrhagic manifestations, pulmonary edema, and oliguria for maternal complications in conjunction with perinatal death, intrauterine growth restriction, preterm birth, neonatal thrombocytopenia, leukopenia, neutropenia, and RDS for fetal complications. However, in the overall analysis, the correlation between improvements in the outcomes of patients with HELLP syndrome following treatment with corticosteroids was not detected for the included population.
As the results demonstrate, although limited, there is no evidence supporting the benefits of corticosteroids administration. Although outcomes of mothers with HELLP syndrome were improved in some included RCTs, with regard to the recovery of normal platelet counts, liver enzymes, and relief from hemolysis, no significant correlations were observed in this pooled meta-analysis. For example, oliguria, an important patient-oriented outcome of the HELLP syndrome, did not differ significantly after treatment with corticosteroids or placebo, while a clinical review revealed that a benefit was observed from the use of corticosteroids to treat oliguria, another complication accompanying HELLP syndrome (Citation28). As for the other included comorbidities, an insignificant correlation was also found, and these differences might be attributable to the limited data available for this review. Previous studies have reported contradictory findings (Citation16,Citation27) regarding neonatal outcomes, and treatment with corticosteroids and placebo did not show significant differences. Whether corticosteroids should be used for treatment remains controversial, as no specific benefits have been reported. Furthermore, with respect to neonates with evident pathological manifestations such as RDS or IVH, a previous study (Citation5) illustrated that neonatal morbidity and death were related to gestational age rather than to the presence or absence of HELLP syndrome, at gestation weeks 24 to 36. In the included RCTs, the neonatal outcomes of gestational age at delivery were 28.4 ± 1.1 and 28.8 ± 1.7 in the corticosteroids and placebo-treated groups, respectively. Premature birth was considered a decisive factor defining neonatal outcomes. Although one study (Citation29) evaluating the effects of antenatal corticosteroids (ACS) treatment on morbidity of preterm newborns revealed that ACS treatment significantly reduced the risk of RDS and IVH in preterm infants. This result may also be attributed to the limited pooled data available on neonatal outcomes. The discrepancies may not occur only by chance across all these trails, additional unknown factors may be involved that warrant further investigation in order to improve treatment decisions for clinicians. Barriers should be overcome and should not represent limitations or restrictions. The mechanisms underlying HELLP syndrome remain unknown, while the role of consanguinity and preeclampsia require further study. In one study, molecular advances in the pathophysiology of preeclampsia and HELLP syndromes were investigated. The study indicated that serum ADAMTS13 activity directly regulated Von Willebrand to induce platelet aggregation, eventually leading to the occlusion of small vessels. Furthermore, the complement cascade was also mentioned, because in women with pre-eclampsia or HELLP syndrome, complement control is interrupted and terminal complement activation is enhanced (Citation13). These two aspects could stimulate future studies to provide further targeted treatment of HELLP syndrome, while many other aspects of HELLP pathophysiology deserve further exploration.
The clinical implications of this study will play an essential role in determining whether corticosteroids should be administered to patients with HELLP syndrome. As HELLP syndrome is a dynamic process characterized by exacerbation and remissions of clinical and biochemical abnormalities, it is also an illness that affects multiple systems. Consequently, multidisciplinary hospital departments must participate in the therapeutic regimen or decisions to improve rehabilitation and even recovery of patients with HELLP syndrome.
Although this review identified a wealth of existing data that could be used to examine the efficacy of corticosteroids in HELLP syndrome, there were some limitations in completing a meta-analysis. Firstly, heterogeneity in the use of platelet count as a variable, for example, the initial measure was not provided in some studies, but a formula was provided, consequently, a data transformation was conducted using the supplied formula, where possible, in order to increase the number of studies to be pooled in the meta-analysis. Furthermore, some crucial data, such as liver enzyme levels, were omitted because the included literature only provided the baseline characteristics of the patients, while the values after corticosteroids administration were not provided, and could have influenced the comprehensiveness of this study. Secondly, there were a limited number of studies in each meta-analysis, which meant that meta-regression could not be used to explore sources of heterogeneity. However, the strengths of the study should also be highlighted. Attempts were made to minimize human error and subjectivity, including duplicate independent screening and quality assessment, and validation of all data extraction. The limited number of studies that could be pooled in each individual meta-analysis also meant that the investigation of publication bias and performing sensitivity analysis was limited. Although the potential factors relative to pregnant women with HELLP syndrome were limited, we added neonatal outcomes as an additional variable, which could also provide an important contribution to improve decision-making among clinicians. All available data in the literature indicated that the decisive factors in evaluating HELLP syndrome were not clear or a numerical transformation was required using the original data. Corticosteroid treatment of patients with HELLP syndrome should consider not only the baseline characteristics of patients, but also the long-term effects after treatment to better address maternal and fetal health and well-being.
Conclusions
This study evaluated the efficacy of corticosteroids treatment in patients with HELLP syndrome, revealing that corticosteroids did not provide a significant benefit in clinical outcomes for pregnant women and newborns with HELLP. High-quality RCTs with larger patient samples are required to provide more definitive conclusions on the role of corticosteroids in patients with HELLP.
Authors’ contributors
JMS designed the research; WJS, JH and QZ collected the data and verified the accuracy of the data. JMS and WJS verified the accuracy of the data; WJS and JH contributed to data interpretation; WJS and QZ performed the statistical analysis and visualization; JMS and WJS wrote the manuscript. The authors read, critically reviewed, and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142(2):159–10. doi: 10.1016/S0002-9378(16)32330-4
- Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A review. Bmc Pregnancy Childbirth. 2009;9(1):8. doi: 10.1186/1471-2393-9-8
- Lam MTC, Dierking E. Intensive care unit issues in eclampsia and HELLP syndrome. Int J Crit Illn Inj Sci. 2017;7(3):136–141. doi: 10.4103/IJCIIS.IJCIIS_33_17
- Sadaf N, Haq G, Shukar-Ud-Din S. Maternal and foetal outcome in HELLP syndrome at tertiary care hospital. J Pak Med Assoc. 2013;63(12):1500–1503.
- Abramovici D, Friedman SA, Mercer BM, et al. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation: does the HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180(1):221–225. doi: 10.1016/S0002-9378(99)70178-X
- Abildgaard U, Heimdal K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):117–123. doi: 10.1016/j.ejogrb.2012.09.026
- Geary M. The HELLP syndrome. Br J Obstet Gynaecol. 1997;104(8):887–891. doi: 10.1111/j.1471-0528.1997.tb14346.x
- Amylidi-Mohr S, Kubias J, Neumann S, et al. Reducing the risk of preterm preeclampsia: comparison of two first trimester screening and treatment strategies in a single centre in Switzerland. Geburtshilfe Frauenheilkd. 2021;81(12):1354–1361. doi: 10.1055/a-1332-1437
- Wallace K, Harris S, Addison A, et al. HELLP syndrome: pathophysiology and Current therapies. Curr Pharm Biotechnol. 2018;19(10):816–826. doi: 10.2174/1389201019666180712115215
- Wallace K, Martin JN Jr., Tam Tam K, et al. Seeking the mechanism(s) of action for corticosteroids in HELLP syndrome: SMASH study. Am J Obstet Gynecol. 2013;208(5):380.e381–388. doi: 10.1016/j.ajog.2013.01.049
- Jiménez KM, Morel A, Parada-Niño L, et al. Identifying new potential genetic biomarkers for HELLP syndrome using massive parallel sequencing. Pregnancy Hypertens. 2020;22:181–190. doi: 10.1016/j.preghy.2020.09.003
- Cam T, Cimilli Senocak GN, Ozturk N, et al. May human epididymis 4 protein play a role in the etiopathogenesis of hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome? J Obstetrics Gynaecol Res. 2021;47(7):2324–2328. doi: 10.1111/jog.14808
- Gardikioti A, Venou T-M, Gavriilaki E, et al. Molecular Advances in Preeclampsia and HELLP Syndrome. Int J Mol Sci. 2022;23(7):3851. doi: 10.3390/ijms23073851
- Ozer A, Kanat-Pektas M, Ozer S, et al. The effects of betamethasone treatment on clinical and laboratory features of pregnant women with HELLP syndrome. Arch Gynecol Obstetrics. 2009;280(1):65–70. doi: 10.1007/s00404-008-0865-3
- Watterberg K, Aucott S, Benitz W, et al. The Apgar score. Pediatrics. 2015;136(4):819–822. doi: 10.1542/peds.2015-2651
- Woudstra DM, Chandra S, Hofmeyr GJ, et al. Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy. Cochrane Database Syst Rev. 2010;2010Cd008148. doi: 10.1002/14651858.CD008148.pub2
- Yang L, Ren C, Mao M, et al. Prognostic factors of the efficacy of High-dose corticosteroid therapy in hemolysis, elevated liver enzymes, and Low platelet count syndrome during pregnancy: a meta-analysis. Medicine. 2016;95(13):e3203. doi: 10.1097/MD.0000000000003203
- Simó R, Hernández C. New insights into treating early and advanced stage diabetic retinopathy. Int J Mol Sci. 2022;23(15):8513. doi: 10.3390/ijms23158513
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898
- Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis. J Clin Psychiatry. 2020;81:20f13681. doi: 10.4088/JCP.20f13681
- Barrilleaux PS, Martin JN Jr., Klauser CK, et al. Postpartum intravenous dexamethasone for severely preeclamptic patients without hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome: a randomized trial. Obstet & Gynecol. 2005;105(4):843–848. doi: 10.1097/01.AOG.0000154887.57440.d1
- Fonseca JE, Méndez F, Cataño C, et al. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005;193(5):1591–1598. doi: 10.1016/j.ajog.2005.07.037
- Fonseca JE, Otero JC, Messa C. Dexamethasone for the treatment of class I HELLP syndrome: a double-blind, placebo-controlled, multicenter, randomized clinical trial. Pregnancy Hypertens. 2019;17:158–164. doi: 10.1016/j.preghy.2019.06.003
- Katz L, de Amorim MM, Figueiroa JN, et al. Postpartum dexamethasone for women with hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2008;198(3):283.e281–288. doi: 10.1016/j.ajog.2007.10.797
- Magann EF, Bass D, Chauhan SP, et al. Antepartum corticosteroids: disease stabilization in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171(4):1148–1153. doi: 10.1016/0002-9378(94)90054-X
- van Runnard Heimel PJ, Huisjes AJ, Franx A, et al. A randomised placebo-controlled trial of prolonged prednisolone administration to patients with HELLP syndrome remote from term. Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):187–193. doi: 10.1016/j.ejogrb.2005.11.041
- Mao M, Chen C. Corticosteroid therapy for management of hemolysis, elevated liver enzymes, and Low platelet count (HELLP) syndrome: a meta-analysis. Med Sci Monit. 2015;21:3777–3783. doi: 10.12659/MSM.895220
- Clenney TL, Viera AJ. Corticosteroids for HELLP (haemolysis, elevated liver enzymes, low platelets) syndrome. BMJ. 2004;329(7460):270–272. doi: 10.1136/bmj.329.7460.270
- Morhart P, Gärtner J, Weiss C, et al. Influence of timing of antenatal corticosteroid administration on morbidity of preterm neonates. Vivo (Athens, Greece). 2022;36(4):1777–1784. doi: 10.21873/invivo.12891