ABSTRACT
Objective
The aim of this study was to investigate the possible causal relationship between COVID-19 and the risk of pre-eclampsia/eclampsia using a Mendelian randomized (MR) design.
Methods
We estimated their genetic correlations and then performed two-sample Mendelian randomization analyses using pooled statistics from the COVID-19 susceptibility/hospitalization genome-wide association study and the pre-eclampsia/eclampsia datasets. The main analyses were performed using the inverse variance weighting method, supplemented by the weighted median method and the MR-Egger method.
Results
We identified a significant and positive genetic correlation between COVID-19 susceptibility and pre-eclampsia/eclampsia [OR = 1.23 (1.01–1.51), p = 0.043]. Meanwhile, hospitalization of COVID-19 was significantly associated with a higher risk of pre-eclampsia/eclampsia [OR = 1.15 (1.02–1.30), p = 0.024]. Consistently, hospitalization of COVID-19 were nominally associated with higher risk of pre-eclampsia [OR = 1.14, (1.01–1.30), p = 0.040]. The results were robust under all sensitivity analyses.
Conclusion
These results suggest that COVID-19 may increase the risk of pre-eclampsia/eclampsia. Future development of preventive or therapeutic interventions should emphasize this to mitigate the complications of COVID-19.
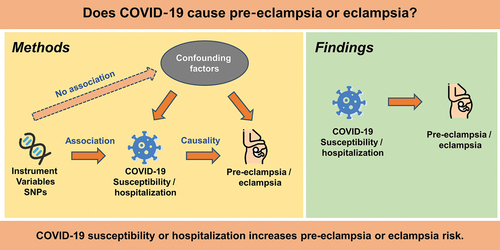
Introduction
Approximately three to five per cent of pregnancies are pre-eclampsia, which causes a minimum 42,000 maternal fatalities on a yearly basis (Citation1,Citation2). Pre-eclampsia is characterized by the onset or progression of hypertension and proteinuria after 20 weeks of pregnancy (Citation3). It may lead eclampsia, which manifests as seizures and can be life-threatening (Citation4). The pathogenesis of pre-eclampsia is not fully understood yet. Several theories have been proposed, including placental ischemia and oxidative stress, endothelial dysfunction and excessive vascular inflammation (Citation2). However, these theories have not been able to completely explain the pathogenesis of pre-eclampsia.
Research has indicated a correlation between the presence of periodontal disease or other infections during pregnancy and a heightened likelihood of developing pre-eclampsia (Citation5,Citation6). Infections lead to impaired maternal blood flow, placental ischemia, and hypoxia. Then increased levels of inflammatory markers and endothelial dysfunction can trigger pre-eclampsia. COVID-19. The global pandemic in recent years has been caused by the viral respiratory illness transmitted by Severe Acute Respiratory Syndrome Corona Virus 2, known as SARS-CoV-2 (Citation7). As a global health emergency, the number of cases and deaths has been on the rise (Citation8). Pregnant women remain at risk of SARS-CoV-2 infection due to vaccine waning over time, immune escape due to viral variants, and weakened immune protection (Citation9). Previous research showed a causal link between COVID-19 infection and an increased risk of hypertensive disorders during pregnancy (Citation10). However, the evidence regarding the association between COVID-19 and pre-eclampsia remains limited, previous study does not further classify the relationship between COVID-19 and pre-eclampsia. Existing research showed a causal relationship between COVID-19 infection and increased risk of pregnancy hypertension, but the evidence of COVID-19 and pre-eclampsia is still absent.
Previous studies that indicated the potential association between pre-eclampsia and COVID-19, the relation of COVID-19 and pre-eclampsia/eclampsia has been reported by observational studies and meta-analyses have reported (Citation9). Nevertheless, confounding variables may have influenced the studies, and causality cannot be established. Mendelian randomization (MR) is a technique for estimating the causal connection between risk factors and outcomes (Citation11,Citation12). It is less affected by confounders and reverse causality than traditional observational studies. Similar to randomized controlled trials (RCT), in which participants are assigned at random (Citation13). Using the GWAS database, we analyzed the possible causal association of susceptibility or hospitalization due to COVID-19 and pre-eclampsia or eclampsia through two-sample MR.
Materials and methods
Study design
Using a two-sample MR, we assessed COVID-19’s causation on pre-eclampsia or eclampsia risk. Data of COVID-19 infection, pre-eclampsia and eclampsia from the public published GWAS datasets were used. Ethical approval was not required for this study, as the data were previously published. illustrates the unbiased causal relationship and hypotheses generated by this MR analysis.
Figure 1. Mendelian randomization assumptions. We selected SNPs associated with COVID-19 and estimated the corresponding effects of these SNPs based on the risk of pre-eclampsia/eclampsia obtained from European populations.
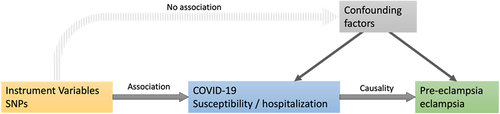
The central idea of Mendelian randomization is that the genotype of a genetic variant determines different intermediate phenotypes, and if the phenotype characterizes an individual’s exposure, then an assessment of the association between genotype and disease should be able to model the effect of exposure on disease. Instrumental variables (IVs) are used to address endogeneity issues such as confounding, measurement bias and temporal confounding. Factors that can be used as instrumental variables must fulfil three conditions: (1) correlation: the IV must be robustly and strongly correlated with the exposure; (2) independence: the IV must be independent of confounders; and (3) exclusivity: the IV must affect the outcome only through the exposure. The SNPs associated with the corresponding phenotypes with statistical significance at the genome-wide level (p < 5 × 10−8) were identified as IVs. Independent inheritance (linkage disequilibrium r2 <0.001, distance window 10,000 kb) was determined for the selected SNPs.
Data source
The GWAS data for COVID-19 were from the IEU open GWAS project (https://www.covid19hg.org/about/). The two data sets of COVID-19 extracted from European Bioinformatics Institute (EBI) included 1,299,010 and 1,887,658 European ancestry individuals, which were susceptibility and hospitalization, respectively (). The phenotype on susceptibility contrasted patients who contracted COVID-19 to the controls. Infection in this case was denoted as having SARS-CoV-2 that was confirmed by digital medical information or patient self-reporting. The hospitalization phenotype compares patients hospitalized with COVID-19 to a control group not hospitalized with COVID-19 or not carrying COVID-19.
Table 1. Data source and profiles.
The genetic data of pre-eclampsia and eclampsia were extracted from the IEU open GWAS project (https://r8.risteys.finngen.fi/). It was obtained from FinnGen of university of Helsinki which comprised a total of 118,638 and 118,291 samples with European ancestry (). The pre-eclampsia and eclampsia diagnosis was established based on the International Classification of Diseases (ICD-10 O11, O14, O15).
Statistical analysis
We utilized principal analysis with the inverse-variance weighted (IVW) approach to analysis COVID-19’s relationship with eclampsia. This was performed through coefficient regression based on a genetic variant outcome against variant exposure, then applying weightages on estimate using the instrument-outcome association’s inverse variances (Citation14). In addition, the median weighted estimate (MME) was also conducted for the results’ stability and reliability. To mitigate horizontal pleiotropy, instrumental variables that were outliers as determined by MR-PRESSO outlier test were eliminated. Examining the heterogeneity of the individual causation using Cochran’s Q. Using MR-Egger, effect due to pleiotropy was further investigated. A leave-one-out analysis on sensitivity was likewise conducted. All analyses were performed by R 4.2.1 with “TwoSampleMR” and “MRPRESSO” packages.
Results
Mendelian randomization analyses for COVID-19
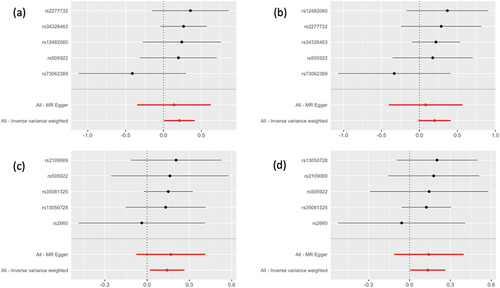
We utilized MR-Egger, IVW, and weighted median regression to approximate the genetically projected COVID-19’s causation on pre-eclampsia or eclampsia. Each genetic instrument’s association with COVID-19 was illustrated in Supplement –4. The two GWAS datasets for pre-eclampsia/eclampsia present the approximate results. The gene predicted a significant positive association between COVID-19 susceptibility and pre-eclampsia or eclampsia [OR = 1.23 (1.01–1.51), p = 0.043]. COVID-19 hospitalization was associated positively with pre-eclampsia and eclampsia risk [finn-b-O15_PRE_OR_ECLAMPSIA: OR = 1.15 (1.02–1.30), p = 0.024; finn-b-O15_PREECLAMPSIA, OR = 1.14 (1.01–1.30), p = 0.040]. While no significant association between COVID-19 susceptibility and pre-eclampsia ().
Table 2. Effects of COVID-19 on the risk of pre-eclampsia/eclampsia in pregnancy by IVW and weighted median methods.
The outcomes of MR-PRESSO analysis revealed no SNPs that were anomalous. The weighted median analysis () produces OR estimates that are similar in direction to conventional MR analysis (IVW) but are less precise. demonstrates that MR-Egger analysis revealed no significant causality.
Figure 2. Forest plot to visualize causal effect of each single SNP on the risk. Results from the MR analysis to evaluate causal role of: (a) COVID-19 susceptibility and pre-eclampsia or eclampsia; (b) COVID-19 susceptibility and pre-eclampsia; (c) COVID-19 hospitalization and pre-eclampsia or eclampsia; (d) COVID-19 hospitalization and pre-eclampsia.
Analysis of horizontal pleiotropy
We performed extensive sensitivity analyses to validate the causal association between COVID-19 and the risk of pre-eclampsia/eclampsia (). The Cochran’s Q test was not identify heterogeneity in effect across IVs. The funnel plot shows the Wald ratios for each SNP, where asymmetry indicates horizontal pleiotropy. Due to fewer IVs, it was difficult to assess horizontal pleiotropy using funnel plots (Supplemental Figure 1). As a result, we conducted additional analyses utilizing the MR Egger intercept and found no directional pleiotropy proof in this research (p > 0.05). Furthermore, the MR-PRESSO analysis did not identify any potential instrumental outliers (p > 0.05).
Table 3. Heterogeneity and horizontal pleiotropy analyses between COVID-19 and pre-eclampsia/eclampsia.
Effects of individual genetic instruments in relation to hypertension disorders in pregnancy
We likewise conducted leave-one-out investigation to determine how each SNP affected the estimate of the general effect. When we disregarded each SNP and did the MR analysis again, the estimated causal effects were not significantly different (Supplemental Figure 2). Therefore, no single genetic tool can account for the estimated effect.
Discussion
Mendelian randomization analysis has been employed in studies to investigate the association between COVID-19 and various diseases, including cardiovascular disease, cancer, neurodegenerative disorders, among others (Citation15–17). A previous two-sample MR study found that genetic predisposition to COVID-19 may increase the risk of hypertension disorders in pregnancy (Citation10), and our study indicated a stronger association between COVID-19 susceptibility and pre-eclampsia/eclampsia. Our study found the association between COVID-19 susceptibility, hospitalization, and pre-eclampsia/eclampsia. We identified the associations between these conditions and several related SNPs (Supplemental Table 1-4). One of the SNPs, rs505922, was significantly associated with severe and hospitalized cases of COVID-19 (Citation18), and a meta-analysis also revealed its relation with cerebrovascular diseases (Citation19). In addition, the other SNPs (rs12482060, rs13050728, rs2109069, rs2277732, rs2660, rs34326463, rs35081325, and rs73062389) were found linked to COVID-19 infection, susceptibility, or critical illness (Citation17,Citation20–22). These findings may potentially lead to improved risk assessment, preventive measures, and therapeutic strategies for pregnant women.
According to previous observational studies, pregnant women with COVID-19 have a higher odds of pre-eclampsia, with ORs ranging from 1.22 to 3.43 across different countries and regions (Citation23–28). Furthermore, a multi-study, large-sample meta-analysis on SARS-CoV-2 infection during pregnancy and risk of pre-eclampsia, including 28 studies and 790,954 subjects showed that the prevalence of pre-eclampsia in pregnant women with COVID-19 was 1.58 times higher than in those without COVID-19 (Citation29). However, one study has results inconsistent with the above, no significant association was found between them, perhaps due to the small sample size as seven samples (Citation30).
In addition, we hypothesized a possible dose–response association between COVID-19 severity and the pre-eclampsia and eclampsia development risk. A few studies assessed the association between COVID-19 severity and pre-eclampsia also validated our results (Citation31–33). Pregnant women with severe COVID-19 have five-times higher pre-eclampsia risk compared to patients without symptoms, according to a study by Lai et al. In addition, at-least moderate COVID-19 infection was associated with a 3,3-fold increased risk of pre-eclampsia compared to at-most mild infections (Citation34). Besides, current evidences have reported COVID-19 vaccination in pregnant can contribute to the prevention of adverse outcomes such as stillbirth and preterm birth (Citation35,Citation36). While some research found no significant difference in the occurrence of pre-eclampsia between COVID-19 vaccination and the control group (Citation37,Citation38).
There were some speculations about the mechanism of SARS-CoV-2 infection and pre-eclampsia. The circulating renin-angiotensin systems (RASs) upon pregnancy regulate fluid volume and blood pressure (Citation39). Angiotensin-converting enzyme 2 (ACE2), a key component of the RAS, primarily mediates the conversion of angiotensin II (Ang II) to angiotensin-(1-7) [A ng(1–7)]. Ang II can induce vasoconstrictor, oxidative stress and inflammation. A ng-(1-7) has opposite effects against it (Citation40). ACE2 is also a receptor for SARS-CoV-2. After they bind on the cell membrane, SARS-CoV-2 will invade the ACE2 binding site or cause ACE2 to fall off (Citation41). This would decreasing the local metabolism of Ang II to Ang-(1-7), which causes inflammation, hypertension, and coagulopathies (Citation42). This is consistent with a pathological explanation of pre-eclampsia (Citation43). A silico study of NCBI-GEO revealed that SARS-CoV-2’s receptors for entry expressed in placenta. Various proteins interacting with COVID-19 are indispensable for placental functions like syncytium formation, differentiation, implantation, and trophoblast invasion and migration. This is consistent with the pathology states of pre-eclampsia (Citation44).
The main strength of the study is that MR analysis is generally less affected by confounding factors. In addition, multiple statistical methods were employed to corroborate the consistency of the results, and a parallel sensitivity analysis was conducted to validate the validity of our findings (Citation45). The study has several limitations. Our findings are primarily based on European populations, preventing us from generalizing them to other ethnic groupings. Second, the susceptibility and hospitalization phenotypes of COVID-19 May be affected by a number of factors, such as medical conditions in each country, that were not taken into consideration in the MR analysis. Third, the samples comprising the COVID-19 data sets were collected before April 2020 and therefore do not include recent waves and variants of SARS-CoV-2 infections.
To validate these findings, further clinical data and a comprehensive study of the possible causal mechanism are required.
Highlights
Used Mendelian randomization to probe COVID-19’s effect on pre-eclampsia/eclampsia.
Employed data from EBI and FinnGen for analysis.
COVID-19 susceptibility/hospitalization increases pre-eclampsia/eclampsia risk by approximately 20%.
Supplemental Material
Download MS Word (234.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
This is publicly available data that can be accessed at: https://www.covid19hg.org/about/, https://r8.risteys.finngen.fi/
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2285757
Additional information
Funding
References
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–7. doi: 10.1016/S2214-109X(14)70227-X
- Chappell LC, Cluver CA, Kingdom J, et al. Pre-eclampsia. Lancet. 2021;398:341–354. doi: 10.1016/S0140-6736(20)32335-710297
- Chaiworapongsa T, Chaemsaithong P, Yeo L, et al. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–480. doi: 10.1038/nrneph.2014.102
- Erez O, Romero R, Jung E, et al. Preeclampsia and eclampsia: the conceptual evolution of a syndrome. Am J Obstet Gynecol. 2022;226(2):S786–S803. doi: 10.1016/j.ajog.2021.12.001
- Konopka T, Zakrzewska A. Periodontitis and risk for preeclampsia — a systematic review. Ginekol Pol. 2020;91(3):158–164. doi: 10.5603/GP.2020.0024
- Nourollahpour SM, Behboodi MZ, Adam I, et al. Human infectious diseases and risk of preeclampsia: an updated review of the literature. Infection. 2017;45(5):589–600. doi: 10.1007/s15010-017-1031-2
- El-Sadr WM, Vasan A, El-Mohandes A. Facing the New covid-19 Reality. N Engl J Med. 2023;388(5):385–387. doi: 10.1056/NEJMp2213920
- WHO Coronavirus (COVID-19) Dashboard. [Last read 20th Aug 2023]. https://covid19.who.int.
- Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet Lond Engl. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0
- Tan J, Liu N, Guo T, et al. Genetic predisposition to COVID-19 may increase the risk of hypertension disorders in pregnancy: a two-sample Mendelian randomization study. Pregnancy Hypertens. 2021;26:17–23. doi: 10.1016/j.preghy.2021.08.112
- Davies NM, Holmes MV, Davey SG. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601
- Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070
- Lawlor DA, Harbord RM, Sterne JAC, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034
- Bowden J, Davey SG, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080
- Li C, Liu J, Lin J, et al. COVID-19 and risk of neurodegenerative disorders: A Mendelian randomization study. Transl Psychiatry. 2022;12(1):283. doi: 10.1038/s41398-022-02052-3
- Li J, Bai H, Qiao H, et al. Causal effects of COVID-19 on cancer risk: a Mendelian randomization study. J Med Virol. 2023;95(4):e28722. doi: 10.1002/jmv.28722
- Miao J, Gu X, Shi R. COVID-19 is associated with the risk of cardiovascular disease death: a two-sample Mendelian randomization study. Front Cardiovasc Med. 2022;9:974944. doi: 10.3389/fcvm.2022.974944
- Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the million veteran program. PLoS Genet. 2022;18(4):e1010113. doi: 10.1371/journal.pgen.1010113
- Li Y, Liu L, Huang Y, et al. Association of ABO polymorphisms and pancreatic Cancer/Cardiocerebrovascular disease: a meta-analysis. BMC Med Genet. 2020;21(1):41. doi: 10.1186/s12881-020-0975-8
- Zhang J, Wu F, Chen S, et al. Genetic predisposition to severe COVID-19 might increase the risk of stroke: a two-sample Mendelian randomization study. Front Genet. 2022;13:895211. doi: 10.3389/fgene.2022.895211
- Velavan TP, Pallerla SR, Rüter J, et al. Host genetic factors determining COVID-19 susceptibility and severity. EBioMedicine. 2021;72:103629. doi: 10.1016/j.ebiom.2021.103629
- dos Santos ACM, dos Santos BRC, dos Santos BB, et al. Genetic polymorphisms as multi-biomarkers in severe acute respiratory syndrome (SARS) by coronavirus infection: a systematic review of candidate gene association studies. Infect Genet Evol. 2021;93:104846. doi: 10.1016/j.meegid.2021.104846
- Brandt JS, Hill J, Reddy A, et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol. 2021;224(4):e389.1–.e389.9. doi: 10.1016/j.ajog.2020.09.043
- Chornock R, Iqbal SN, Wang T, et al. Incidence of hypertensive disorders of pregnancy in women with COVID-19. Am J Perinatol. 2021;38(8):766–772. doi: 10.1055/s-0041-1727167
- Gurol-Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225(5):.e522.1–.e522.11. doi: 10.1016/j.ajog.2021.05.016
- Grechukhina O, Greenberg V, Lundsberg LS, et al. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am J Obstet Gynecol MFM. 2020;2(4):100246. doi: 10.1016/j.ajogmf.2020.100246
- Yang R, Mei H, Zheng T, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18(1):330. doi: 10.1186/s12916-020-01798-1
- Prabhu M, Cagino K, Matthews K, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG Int J Obstet Gynaecol. 2020;127(12):1548–1556. doi: 10.1111/1471-0528.16403
- Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226(1):68–89.e3. doi: 10.1016/j.ajog.2021.07.009
- Pirjani R, Hosseini R, Soori T, et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med. 2020;27(7):taaa158. doi: 10.1093/jtm/taaa158
- Sánchez J, Espinosa J, Caballero LC, et al. COVID 19 and high pregnancy and perinatal complications in Panama. J Matern Fetal Neonatal Med. 2022;35(25):8245–8248. doi: 10.1080/14767058.2021.1967925
- Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137(4):571–580. doi: 10.1097/AOG.0000000000004339
- Mahajan NN, Kesarwani S, Kumbhar P, et al. Increased risk of early-onset preeclampsia in pregnant women with COVID-19. Hypertens Pregnancy. 2023;42(1):2187630. doi: 10.1080/10641955.2023.2187630
- Lai J, Romero R, Tarca AL, et al. SARS-CoV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose-response relationship supporting causality. Am J Obstet Gynecol. 2021;225(6):689–693.e1. doi: 10.1016/j.ajog.2021.08.020
- Prasad S, Kalafat E, Blakeway H, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. 2022;13(1):2414. doi: 10.1038/s41467-022-30052-w
- Shafiee A, Gargari OK, Athar MMT, et al. COVID-19 vaccination during pregnancy: a systematic review and meta-analysis. Bmc Pregnancy Childbirth. 2023;23(1):45. doi: 10.1186/s12884-023-05374-2
- Theiler RN, Wick M, Mehta R, et al. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. doi: 10.1016/j.ajogmf.2021.100467
- Piekos SN, Hwang YM, Roper RT, et al. Effect of COVID-19 vaccination and booster on maternal–fetal outcomes: a retrospective cohort study. Lancet Digit Health. 2023;5(9):e594–e606. doi: 10.1016/S2589-7500(23)00093-6
- Irani RA, Xia Y. The functional role of the renin–angiotensin System in pregnancy and preeclampsia. Placenta. 2008;29(9):763–771. doi: 10.1016/j.placenta.2008.06.011
- Khlestova GV, AYu R, Nizyaeva NV, et al. Dynamics of renin, angiotensin II, and angiotensin (1–7) during pregnancy and predisposition to hypertension-associated complications. Bull Exp Biol Med. 2018;165(4):438–439. doi: 10.1007/s10517-018-4188-5
- Li G, He X, Zhang L, et al. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun. 2020;112:102463. doi: 10.1016/j.jaut.2020.102463
- Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open. 2020;4(02):e138–e144. doi: 10.1055/s-0040-1713678
- Magee LA, Nicolaides KH, von Dadelszen PP, et al. Preeclampsia. N Engl J Med. 2022;386(19):1817–1832. doi: 10.1056/NEJMra2109523
- Seethy AA, Singh S, Mukherjee I, et al. Potential SARS-CoV-2 interactions with proteins involved in trophoblast functions – an in-silico study. Placenta. 2021;103:141–151. doi: 10.1016/j.placenta.2020.10.027
- Richmond RC, Smith GD. Mendelian randomization: concepts and scope. Cold Spring Harb Perspect Med. 2022;12(1):a040501. doi: 10.1101/cshperspect.a040501

