?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Hypertensive disorders in pregnancy (HDP) are a major cause of maternal mortality and morbidity. Recent studies indicated that pregnant women are the most vulnerable populations to ambient temperature influences, but it affected HDP with inconsistent conclusions. Our objective is to systematically review whether extreme temperature exposure is associated with a changed risk for HDP. Method: We searched PubMed, EMBASE, Web of Science and Cochrane Library databases. We included cohort or case control studies examining the association between extreme temperature exposure before or during pregnancy and HDP. Heat sources such as saunas and hot baths were excluded. We pooled the odds ratio (OR) to assess the association between extreme temperature exposure and preeclampsia or eclampsia. Results: Fifteen studies involving 4,481,888 patients were included. Five studies were included in the meta-analysis. The overall result demonstrated that in the first half of pregnancy, heat exposure increases the risk of developing preeclampsia or eclampsia and gestational hypertension, and cold exposure decreases the risk. The meta-analysis revealed that during the first half of pregnancy, heat exposure increased the risk of preeclampsia or eclampsia (OR 1.54, 95% confidence interval (CI): 1.10, 2.15), whereas cold exposure decreased the risk (OR 0.90, 95% CI: 0.84, 0.97). Conclusion: The ambient temperature is an important determinant for the development of HDP, especially for preeclampsia or eclampsia. The effects of extreme temperatures may be bidirectional during the different trimesters of pregnancy, which should be evaluated by future studies. This review provided hints of temperature regulation in HDP administration.
Introduction
Hypertensive disorders in pregnancy (HDP), which are the most common medical complications of pregnancy, occur in 10–15% of all pregnancies worldwide (Citation1–3). HDP include four subtypes, namely, gestational hypertension, preeclampsia or eclampsia, preeclampsia superimposed on chronic hypertension, and chronic hypertension (Citation4). They are a major cause of maternal and offspring morbidity and mortality, especially in low-income and middle-income settings (Citation5,Citation6). The short-term and long-term outcomes of HDP include a preterm delivery for the mother and small for gestational age, stillbirth, and future neurological, cardiovascular, renal and endocrine disorders for the offspring (Citation7–17). The etiology of HDP remain incompletely clear. Previous studies have reported that seasonal change and extreme temperature are important factors for HDP occurrence.
With the aggravation of global climate change in recent years, it has been documented that pregnant women with developing fetuses and young children are considered the populations that are the most vulnerable to environmental influences (Citation18). Studies that have evaluated the influence of the environment on the perinatal outcomes have used the season as an important variable (Citation19). Because the season itself includes a variety of confounding factors, such as the temperature, humidity, and air quality, recent studies have preferred to explore the impact of a single factor, such as the temperature, on the perinatal outcomes. Two recent systematic reviews showed that global warming could increase the incidence of preterm birth, low birth weight, and stillbirth (Citation20,Citation21). 2,3However, the effects of the temperature changes on the HDP have not been systematically recognized.
As the most common disease in pregnancy, HDP is considered a special cardiovascular disease that occurs during the perinatal period. It is particularly important to identify the risk factors for HDP and to further improve the perinatal outcomes for mothers and infants. A temperature driver, which is independent of other environmental factors, of HDP may exist (Citation22). Some evidence had indicated that ambient temperature affected HDP (Citation23,Citation24). However, the relationship between HDP and the ambient temperature has been inconsistent in current studies (Citation25–27). The aim of this review is to systematically assess the associations between the temperature exposure and occurrence of HDP.
Materials and methods
Study selection
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines and was registered in the PROSPERO platform (registration number is CRD42021227878). In brief, we searched Medline (PubMed), EMBASE, Web of Science and Cochrane Library in December 2022. ENDNOTE X7 was used as a platform for screening of the titles, abstracts, and full text articles. Screening of the titles, abstracts, and description/MeSH headings was performed independently by two reviewers (YX M and YZ), with any differences reconciled by a third reviewer (TX).
All of the studies regarding temperature or season exposure were screened to identify the association between the temperature exposure and HDP. The reference lists of the included articles were also screened. The authors were contacted in order to obtain additional information.
HDP has been classified into four categories: 1) gestational hypertension, defined as the onset of hypertension (blood pressure ≥ 140/90 mmHg) after 20 weeks of gestation in previously normotensive women; 2) preeclampsia or eclampsia, diagnosed when hypertension and significant proteinuria develop after the 20th gestational week; 3) preeclampsia superimposed on chronic hypertension, diagnosed as a worsening of hypertension after 20 weeks and with the new development of proteinuria or end organ dysfunction; and 4) chronic hypertension, existing prior to conception or diagnosed before the 20th week of pregnancy (Citation4).
We included studies that met the following criteria: (i) studies examining the incidence of HDP in pregnant women who were exposed to cold/heat temperature before or during pregnancy, as compared to a reference temperature; (ii) cohort study or case control study designs; (iii) studies published in English across all countries; (iv) no restrictions on the published date; and (v) studies that had sufficient information to estimate the outcome. We excluded studies (i) on heat sources such as saunas and hot baths; (ii) systematic reviews, meeting abstracts, letters, editorials, comments, guidelines, and case reports; and (iii) studies that were duplicates or without the adequate outcome measures that we included.
Among all studies that met the inclusion criteria, those that reported effect estimates and could be combined into effect sizes were included in the meta-analysis.
Extracting data, collating, summarizing, and reporting results
Eligible articles were extracted independently in duplicate by two reviewers (YX M and YZ), and all discrepancies were reconciled by the third reviewer (TX). The following data were extracted: authors, year of publication, year(s) of study, study design, countries, sample size, subtypes and definition of HDP. The results were organized into a table that summarized the data, and the table was used to describe the temperature source and measurements, adjusted factors, outcome estimates, and other settings. We did not extract data regarding the impacts of humidity, air pollution or race/ethnicity.
This review presents the primary findings with a summary (14 of 15 studies on temperature exposure and HDP had a significant association). In addition, tables and figures were constructed in order to show the correlation between HDP and a heat or cold temperature during the pregnancy exposure time window. We attempted to identify the particular critical time window (gestational weeks) that had the largest impacts. These data were tabulated and calculated with an Excel spreadsheet (Microsoft).
Quality of study and publication bias
The quality of the cohort or case – control studies was assessed using the Newcastle – Ottawa Scale (NOS)(Citation28. This scale evaluates the selection of study groups (one star for each term), comparability (up to two stars) and exposure or outcome (one star for each term). A low score indicates a high risk of bias. The study quality was classified into the following three categories: high quality (scores 7–9), moderate quality (scores 4–6) and low quality (scores 0–3). We planned to use funnel plots to test for the presence of a publication bias, and Egger’s linear regression was applied to test for funnel plot asymmetry.
Statistical analysis
The effect estimates were extracted from the tables, figures or published textual descriptions in articles or supplementary materials. For studies that presented effect estimate sizes only in a graph, we extracted the effect estimates and the corresponding 95% CI associated with the reference temperature using the WebPlotDigitizer tool (Citation29). Since studies come from different countries or regions, the standards for extreme and reference temperature are quite diverse. According to the included original studies, when extremely hot corresponds to temperatures above the 90th or 95th percentile, and extremely cold corresponds to temperatures below 10th or 5th percentile, the median temperature is used as the reference temperature (Citation22,Citation30,Citation31). When using specific Celsius temperature as the baseline temperature, extreme temperature is defined as an increase or decrease temperature, with the baseline temperature itself serving as the reference temperature (Citation22,Citation25,Citation26). Therefore, the studies of different outcome groups would be quantitatively pooled by following two types of temperature comparisons: “heat/cold temperature vs. reference temperature” and “1°C increase/decrease vs. reference temperature – using the following formula: OR = (ORx)1/x, where x is the increment/reduction of temperature (for example, x = 2°C) for which ORx is stated in the original study (Citation32). ” This allowed us to quantitatively pool estimates from different studies.
A random effects model was used in the meta-analysis to calculate the pooled estimates for different temperature exposures. The effect estimate was assessed with odds ratio (OR) and 95% confidence intervals (CI), and the P values less than 0.05 were considered statistically significant. The I2 statistic was used to quantify the heterogeneity of meta-analysis (I2 >50% is considered substantial heterogeneity) (Citation33). Sensitivity analysis was conducted to test the influence of every study by omitting each estimate. Publication bias was assessed by funnel plot and Egger’s tests (Citation34), and estimated the number of studies missing by the TRIM and FILL method (Citation35). Data was performed using the Stata software (17.0 version; Stata Corp, College Station, TX).
Results
The literature selection and study characteristics
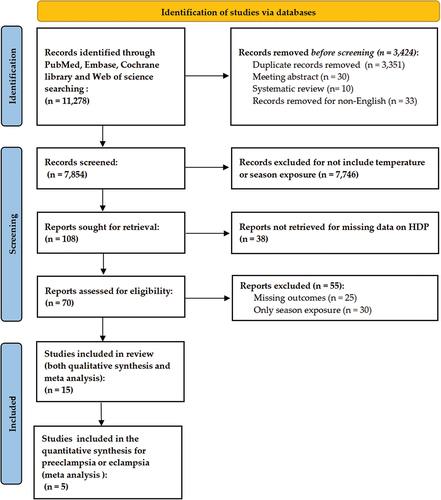
There were 11,278 records identified (PRISMA flowchart, ). We excluded 3,424 studies before the screening. Among the 7,854 records that were screened, 7,746 studies that did not include the influence of the temperature or season exposure on HDP were excluded, and 38 studies were also excluded for missing data. A total of 70 full-text articles were assessed for eligibility, and 55 were excluded due to missing outcomes (n = 25) or missing the ambient temperature as an exposure (n = 30). In total, 15 of these met our study criteria. Five articles complied with the inclusion criteria for the meta-analysis after additional selection (Citation22,Citation25,Citation26,Citation30,Citation31).
For the study characteristics (), 13 of 15 were cohort studies (Citation22,Citation25,Citation27,Citation30,Citation31,Citation36–43), and two were case control studies (Citation26,Citation44). All studies were retrospective in design. A total of 4,481,888 patients were analyzed, with a range of 840 to 2,043,182 women who were analyzed per study. The studies were from 13 countries, eight of which (61.5%) were countries with low or middle incomes (Citation22,Citation27,Citation30,Citation31,Citation37–39,Citation41–43). The temperature exposure data, statistical methods and results of the included studies were summarized in . Eight articles had adjusted for demographic or meteorological variables (maternal age, preexisting diabetes, parity, socioeconomic deprivation, humidity and air pollution) (Citation22,Citation25,Citation26,Citation30,Citation31,Citation39,Citation40,Citation44). Most of the studies (60.0%) found that the temperature (heat or cold exposure was included) was associated with the risk of preeclampsia or eclampsia (9 studies) (Citation25–27,Citation31,Citation38–40,Citation42,Citation43), whereas a few studies showed significant differences between the other subtypes of HDP (Citation22,Citation30).
Table 1. Characteristics of the included studies on ambient temperature and HDP.
Table 2. Summary of temperature exposure data, statistical methods and results.
According to the NOS, the overall methodological quality was good because all of the studies were of high quality, and an additional file shows this in more detail (Supplementary file 1). The NOS quality assessment ranged from 7 to 9 ( = 8.07). Seven articles (score of 7) had points that were detected because their adjusted factors were unclear (Citation27,Citation36–38,Citation41–43).
Heat exposure and the HDP subgroups in the different trimesters of pregnancy
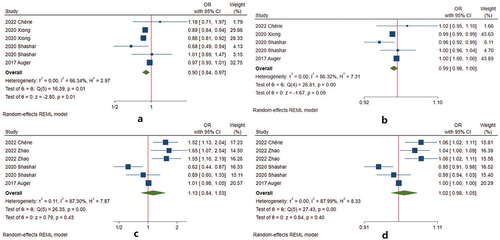
Fourteen studies analyzed heat exposure and the risk of HDP development () (Citation22,Citation25–27,Citation30,Citation31,Citation36–43). For preeclampsia or eclampsia, there is no significant association during p12 (12 weeks before conception) of pregnancy (Citation22). Heat exposure increased the risk during w1–20 (the first half of pregnancy) (Citation22,Citation25–27,Citation30,Citation31,Citation39). Four studies had eligible data for conducting meta-analysis () (Citation22,Citation25,Citation26,Citation30). The pooled estimate was 1.54 (95% CI 1.10–2.15) (). When the studies reported the risk for every 1°C increase, the pooled estimate was 1.07 (95% CI 1.02–1.12) (). After 20 weeks of gestation, there was no consistent conclusion: seven studies found that heat exposure decreased the risk (Citation26,Citation30,Citation31,Citation40–43), two studies showed the opposite conclusion (heat exposure increased the risk) (Citation25,Citation38), and one study found no association (Citation27). The pooled estimate from four studies was 1.05 (95% CI 0.67–1.64) () (Citation25,Citation26,Citation30,Citation31). Every 1°C increase, the OR was 1.01 (95% CI 0.96–1.06) ().
Figure 2. Analysis of the associations between heat exposure and HDP by the gestational weeks of pregnancy. Green shading indicates the period of increased risk during pregnancy after heat exposure. Orange shading indicates the studies that showed a decreased risk. Yellow shading indicates that heat exposure and HDP have no association.
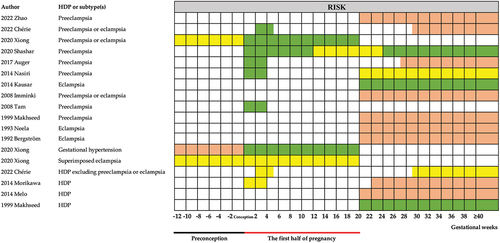
Figure 3. Meta-analysis of the associations between heat exposure and preeclampsia or eclampsia by the gestational weeks of pregnancy. (a) heat temperature during w1–20 vs. reference temperature; (b) 1°Cincrease during w1–20 vs. reference temperature; (c) heat temperature after 20 weeks of gestation vs. reference temperature; (d) 1°Cincrease during after 20 weeks of gestation vs. reference temperature.
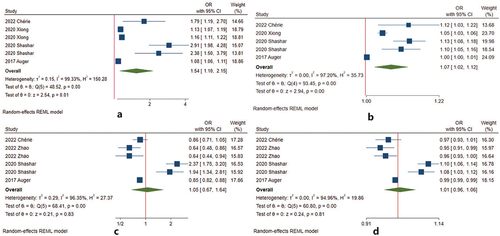
Table 3. Meta-analysis results between extreme temperature exposure and preeclampsia or eclampsia during the gestational weeks.
In addition, heat exposure decreased the risk of gestational hypertension during p12 and increased the risk of gestational hypertension during w1–20 (Citation22). For superimposed preeclampsia or HDP excluding preeclampsia or eclampsia no significant association was observed throughout the pregnancy (Citation22,Citation30).
However, three studies that combined the different subgroups of HDP found inconsistent conclusions between heat exposure and HDP (Citation36,Citation37,Citation41).
Cold exposure and the HDP subgroups in the different trimesters of pregnancy
The association between cold exposure during pregnancy and HDP was reported in all 15 studies () (Citation22,Citation25–27,Citation30,Citation31,Citation36–44). For preeclampsia or eclampsia, cold exposure increased the risk during preconception (Citation22), whereas it decreased the risk during w1–20 (Citation22,Citation25–27,Citation39). We conducted a meta-analysis on four studies with eligible data during w1–20 () (Citation22,Citation25,Citation26,Citation30). The pooled effect of cold exposure was 0.90 (95% CI 0.84–0.97) (). For every 1°C decrease, the pooled estimate was 0.99 (95% CI 0.98–1.00) (). After 20 weeks of gestation, there was no consistent conclusion. Seven studies reported that cold exposure increased the risk (Citation26,Citation30,Citation31,Citation40–43), two studies found that cold exposure decreased the incidence of preeclampsia or eclampsia (Citation25,Citation38), and the remaining two articles found that there was no association (Citation27,Citation44). The pooled effect of cold exposure was 1.13 (95% CI 0.84–1.53) (). For every 1°C decrease, the pooled estimate was 1.02 (95% CI 0.98–1.05) () (Citation25,Citation26,Citation30,Citation31).
Figure 4. Analysis of the associations between cold exposure and HDP by the gestational weeks of pregnancy. Green shading indicates the period of increased risk during pregnancy after cold exposure. Orange shading indicates the studies that showed a decreased risk. Yellow shading indicates that the temperature and HDP have no association.
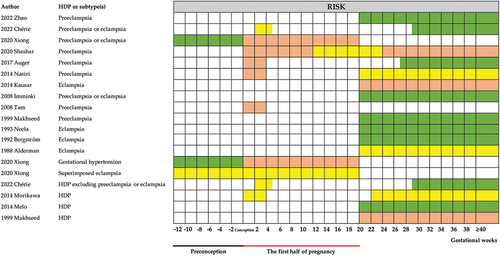
Figure 5. Meta-analysis of the associations between cold exposure and preeclampsia or eclampsia by the gestational weeks of pregnancy. (a) cold temperature during w1–20 vs. reference temperature; (b) 1°Cdecrease during w1–20 vs. reference temperature; (c) cold temperature after 20 weeks of gestation vs. reference temperature; (d) 1° decrease during after 20 weeks of gestation vs. reference temperature.
Cold exposure also increased the risk of gestational hypertension during preconception and decreased the risk during w1–20 (Citation22). No significant association was observed between cold exposure and superimposed preeclampsia (Citation22). For HDP excluding preeclampsia or eclampsia, cold exposure increased the risk after 20 weeks of gestation, no significant association was observed during w1–20 (Citation30).
The studies that combined the different subgroups of HDP had inconsistent conclusions for the association between cold exposure and HDP (Citation36,Citation37,Citation41).
Sensitivity analyses and publication bias diagnostics
Sensitivity analyses showed that excluding any study did not change the overall estimates for temperature exposure, indicating that the results were robust (Supplementary file 2). Next, we evaluated the possibility of publication bias. The funnel plot was roughly symmetrical (Supplementary file 3) and Egger test (heat exposure: p = 0.187; cold exposure: p = 0.319) showed no evidence of publication bias in the association of heat or cold exposure with preeclampsia or eclampsia.
Discussion
In this review, 15 studies involving more than 4 million patients from diverse countries appeared to support the significant association of temperature exposure with HDP (). Most of the studies found a statistically significant risk of HDP after heat or cold temperature exposure ().
Overall, during preconception (p12), cold exposure increases the risk of developing preeclampsia or eclampsia or gestational hypertension, whereas heat exposure decreases the risk of gestational hypertension. In contrast, heat exposure increases the risk of developing preeclampsia or eclampsia or gestational hypertension, and cold exposure decreases the risk of preeclampsia or eclampsia and gestational hypertension during the first half of pregnancy (during w1–20). Meta-analysis of preeclampsia or eclampsia during the first half of pregnancy showed that heat exposure and per 1°C increase caused the higher risk, whereas cold exposure had the lower risk. Interestingly, cold versus heat exposure had opposite effects on preeclampsia or eclampsia and gestational hypertension. Therefore, the effects of extreme temperatures may be bidirectional. Appropriate temperatures may be important to decrease the incidence of preeclampsia or eclampsia and gestational hypertension.
After comparing these risks during preconception with the risks during the first half of pregnancy, the effect of temperature exposure on preeclampsia or eclampsia and gestational hypertension was opposite in the different time windows (Citation22). Cold exposure increased the risk during preconception, which was consistent with the studies that evaluated blood pressure in the general (non-pregnant) population (Citation45,Citation46). However, during w1–20, heat exposure increased the risk. The explanations are as follows: as the mother gains weight and as the fetus grows, pregnant women decrease their capacity for heat loss and increase their internal heat production (Citation47). Thus, heat exposure, which could disturb thermoregulation, induces the activation of the sympathetic nervous system and increases the risk of preeclampsia or eclampsia and gestational hypertension. Future studies need to further define the exact mechanism and confirm these effects.
We found that there was no consistent conclusion for extreme temperature and HDP after 20 weeks of gestation, this may due to different adjusted factors in individual studies. The interaction among meteorological factors may affect the results of the study. Nine studies that were published between 1988 and 2014 detected the influence of meteorological factors on HDP (Citation27,Citation36–38,Citation40–44). These studies did not adjust for other meteorological factors when exploring the impact of temperature exposure on HDP subgroups. Temperature works in concert with other meteorological variables, including air pollution, latitude and sunlight, and can affect people’s health (Citation45,Citation46). This may be one of the important reasons for the heterogeneity of the results in these studies because of the complexity of the interactions between the temperature and other meteorological variables. Three recent studies (2017–2022) explored the association between ambient temperature exposure and subgroups of HDP and considered the other meteorological variables as confounding factors (Citation26,Citation30,Citation31). They drew a consistent conclusion that heat exposure increased the incidence of preeclampsia or eclampsia and cold exposure decreased the risk after 20 weeks of gestation after adjusting for the other meteorological variables. Therefore, the associations between ambient temperature exposure (after 20 weeks of gestation) and preeclampsia or eclampsia need to be proven by more studies with appropriate design.
In this review, we found that temperature exposure has unequal effects in different periods of pregnancy. However, all included studies only explored a certain timeframe within the pregnancy rather than the whole pregnancy. Only one study detected the impact of temperature during preconception and found a significant effect (Citation22). In addition, the effects of temperature exposure may be a long-term process rather than a specific short period of time. The critical time window for extreme temperature exposure remains unknown. Therefore, future studies that use multiple temperature exposures (short- and long-term exposure) during the whole pregnancy period may be necessary.
One question should also be raised regarding whether the temperature plays the same role in the different subtypes of HDP. Our previous study detected multiple subtypes of HDP (preeclampsia or eclampsia, gestational hypertension and superimposed preeclampsia) at the same time. The risk of preeclampsia or eclampsia and gestational hypertension was influenced by the ambient temperature, rather than superimposed preeclampsia (Citation22). Two study found that preeclampsia or eclampsia and other subtypes HDP showed different reactions to extreme temperature exposure (Citation30,Citation41). This may be the reason why the studies combining the different subgroups of HDP had inconsistent conclusions (Citation36,Citation37,Citation41). Future studies should address these differences by separately evaluating the influence of temperature with regard to the individual subtype of HDP.
This review has several limitations, which are as follows. First, the conclusion regarding the impact of cold or heat exposure during the preconception period is based on the data from a single study, highlighting the need for more evidence to support this conclusion. Future studies should explore the relationship between temperature and HDP during the preconception period. Similarly, in the first half of the pregnancy, further investigations are necessary as many of the studies included in our analysis had limited coverage, often spanning only a couple of weeks of exposure. A more comprehensive examination of temperature effects in the first half of pregnancy is warranted. Second, we only included English studies, this may cause biased because of the limited number of trials in the existing literature. The exposure measurement standards were not completely uniform in the individual studies, such as the definition of extreme temperature and the time window of the exposure, which may contribute to the heterogeneity. Third, the sensitivity to temperature may be difference in the different subgroups of people. Our previous study found vulnerable subpopulations among mothers who were aged 20–34 years, were highly educated, had singleton births, had low parity, did not have preterm infants, did not have SGA infants, and lived in urban areas (Citation22). Further studies could clarify the susceptibility for extreme temperature in different populations, in order to prevent vulnerable populations from extreme temperature events. Fourth, another temperature variable, indoor temperature, was not evaluated in these studies. The indoor temperatures are affected by the indoor heating system or air conditioning. The effect of indoor temperatures on the risk of HDP should not be ignored, especially under extreme weather conditions. Evaluating the effect of the indoor temperature should be considered in future studies. Furthermore, the influence of the individual’s behavior (the duration outdoors) should be taken into account in future studies to improve the reliability and comparability of the research evidence.
Conclusion
Ambient temperature is an important determinant of HDP, especially for preeclampsia or eclampsia. The effects of extreme temperatures may be bidirectional during the different trimesters of pregnancy. Appropriate ambient temperatures may be important for pregnant women to avoid potential risk of HDP. The study provided hints of temperature regulation in HDP administration, which should be evaluated by future study. Considering the increased incidence of climate extremes and the significant burden of HDP on human health, research and policy in this area is a high priority.
Abbreviations
| HDP | = | Hypertensive Disorders in Pregnancy |
| NOS | = | Newcastle-Ottawa Scale |
| ACOG | = | American College of Obstetricians and Gynecologists |
| ISSHP | = | International Society for the Study of Hypertension in Pregnancy |
| ICD | = | International Classification and Disease |
| CI | = | confidence interval |
| OR | = | odds ratio |
| RR | = | risk ratio |
Supplemental Material
Download Zip (169.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article because there is no new data was created or analyzed.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2288586
Additional information
Funding
References
- Shah S, Gupta A. Hypertensive disorders of pregnancy. Cardiol Clin. 2019;37(3):345–13. doi: 10.1016/j.ccl.2019.04.008
- Malik R, Kumar V. Hypertension in pregnancy. Advanc exper med biol. 2017;956:375–393.
- Magee LA, Pels A, Helewa M, et al. The hypertensive disorders of pregnancy (29.3). Best Pract Res Clin Obstet Gynaecol. 2015;29(5):643–657.
- Gynecol O. Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016;387(10022):999–1011.
- Bokslag A, van Weissenbruch M, Mol BW, et al. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. doi: 10.1016/j.earlhumdev.2016.09.007
- Bergman L, Bergman K, Langenegger E, et al. PROVE—pre-eclampsia obstetric adverse events: establishment of a biobank and database for pre-eclampsia. Cells. 2021;10(4):959.
- Chen S, Leeton L, Castro J, et al. Myocardial tissue characterisation and detection of myocardial oedema by cardiovascular magnetic resonance in women with pre-eclampsia: a pilot study. Int J Obstet Anesth. 2018;36:56–65. doi: 10.1016/j.ijoa.2018.07.004
- Crispi F, Rodríguez-López M, Bernardino G, et al. Exercise capacity in young adults born small for gestational age. JAMA Cardiol. 2021;6(11):1308.
- Ersbøll A, Bojer A, Hauge M, et al. Long-term cardiac function after peripartum cardiomyopathy and preeclampsia: a Danish nationwide, clinical follow-up study using maximal exercise testing and cardiac magnetic resonance imaging. J Am Heart Assoc. 2018;7(20):e008991.
- Goss KN, Haraldsdottir K, Beshish AG, et al. Association between preterm birth and arrested cardiac growth in adolescents and young adults. JAMA Cardiol. 2020;5(8):910–919.
- Kalapotharakos G, Salehi D, Steding-Ehrenborg K, et al. Cardiovascular effects of severe late-onset preeclampsia are reversed within six months postpartum. Preg Hypert. 2020;19:18–24. doi: 10.1016/j.preghy.2019.12.005
- Kaminski M, Steel K, Jerosch-Herold M, et al. Strong cardiovascular prognostic implication of quantitative left atrial contractile function assessed by cardiac magnetic resonance imaging in patients with chronic hypertension. J Cardiovasc Magn Reson. 2011;13(1):42.
- Mohamed A, Marciniak M, Williamson W, et al. Association of systolic blood pressure elevation with disproportionate left ventricular remodeling in very preterm-born young adults: the preterm heart and elevated blood pressure. JAMA Cardiol. 2021;6(7):821–829.
- Orabona R, Sciatti E, Vizzardi E, et al. Ultrasound evaluation of left ventricular and aortic fibrosis after pre-eclampsia. Ultrasound Obstet Gynecol. 2018;52(5):648–653.
- Siepmann T, Boardman H, Bilderbeck A, et al. Long-term cerebral white and gray matter changes after preeclampsia. Neurology. 2017;88(13):1256–1264.
- Timpka S, Macdonald-Wallis C, Hughes AD, et al. Hypertensive disorders of pregnancy and offspring cardiac structure and function in adolescence. J Am Heart Assoc. 2016;5(11). doi: 10.1161/JAHA.116.003906
- Costello A, Abbas M, Allen A, et al. Managing the health effects of climate change: lancet and University College London institute for global health commission. Lancet (London, England). 2009;373(9676):1693–1733.
- Rohr Thomsen C, Brink Henriksen T, Uldbjerg N, et al. Seasonal variation in the hypertensive disorders of pregnancy in Denmark. Acta Obstet Gynecol Scand. 2020;99(5):623–630. doi: 10.1111/aogs.13786
- Chersich M, Pham M, Areal A, et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ. 2020;371:m3811. doi: 10.1136/bmj.m3811
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3(6):e208243.
- Xiong T, Chen PR, Mu Y, et al. Association between ambient temperature and hypertensive disorders in pregnancy in China. Nat Commun. 2020;11(1). doi: 10.1038/s41467-020-16775-8
- Beltran AJ, Wu J, Laurent O. Associations of meteorology with adverse pregnancy outcomes: a systematic review of preeclampsia, preterm birth and birth weight. Int J Environ Res Public Health. 2013;11(1):91–172. doi: 10.3390/ijerph110100091
- Cil G, Cameron TA. Potential climate change health risks from increases in heat waves: abnormal birth outcomes and adverse maternal health conditions. Risk Anal. 2017;37(11):2066–2079. doi: 10.1111/risa.12767
- Shashar S, Kloog I, Erez O, et al. Temperature and preeclampsia: epidemiological evidence that perturbation in maternal heat homeostasis affects pregnancy outcome. PLoS One. 2020;15(5):e0232877.
- Auger N, Siemiatycki J, Bilodeau-Bertrand M, et al. Ambient temperature and risk of preeclampsia: biased association? Paediatr Perinat Epidem. 2017;31(4):267–271.
- Nasiri R, Shadmehri AA, Ghiassi PK, et al. Association of meteorological factors and seasonality with preeclampsia: a 5-year study in northeast of Iran. Ann Clin Exp Hypertens. 2014;36(8):586–589.
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Rohatgi A. WebPlotDigitizer: web based tool to extract data from plots, images, and maps. Version 3.12. 2017 https://automeris.io/WebPlotDigitizer/.
- Part C, le Roux J, Chersich M, et al. Ambient temperature during pregnancy and risk of maternal hypertensive disorders: a time-to-event study in Johannesburg, South Africa. Environ Res. 2022;212(Pt D):113596.
- Zhao T, Long W, Lu P. Short-term effects of ambient temperature on the risk of preeclampsia in Nanjing, China: a time-series analysis. Bmc Pregnancy Childbirth. 2022;22(1):539. doi: 10.1186/s12884-022-04859-w
- Fatima SH, Rothmore P, Giles LC, et al. Extreme heat and occupational injuries in different climate zones: a systematic review and meta-analysis of epidemiological evidence. Environ Int. 2021;148:106384. doi: 10.1016/j.envint.2021.106384
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629
- Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medic (Baltimore). 2019;98(23):e15987. doi: 10.1097/MD.0000000000015987
- Morikawa M, Yamada T, Yamada T, et al. Seasonal variation in the prevalence of pregnancy-induced hypertension in Japanese women. J Obstetrics Gynaecol Res. 2014;40(4):926–931.
- Melo B, Amorim M, Katz L, et al. Hypertension, pregnancy and weather: is seasonality involved? Rev Assoc Med Bras. 2014;60(2):105–110. doi: 10.1590/1806-9282.60.02.006
- Malik A, Kausar S, Bashir A, et al. Seasonal trends in the occurrence of eclampsia. J SAFOG. 2014;6(2):83–87. doi: 10.5005/jp-journals-10006-1277
- Tam WH, Sahota DS, Lau TK, et al. Seasonal variation in pre-eclamptic rate and its association with the ambient temperature and humidity in early pregnancy. Gynecol Obstet Invest. 2008;66(1):22–26. doi: 10.1159/000114252
- Immink A, Scherjon S, Wolterbeek R, et al. Seasonal influence on the admittance of pre-eclampsia patients in Tygerberg Hospital. Acta Obstet Gynecol Scand. 2008;87(1):36–42. doi: 10.1080/00016340701743066
- Makhseed M, Musini VM, Ahmed MA, et al. Influence of seasonal variation on pregnancy-induced hypertension and/or preeclampsia. Aust NZ J Obstetrics Gynaecol. 1999;39(2):196–199. doi: 10.1111/j.1479-828X.1999.tb03372.x
- Neela J, Raman L. Seasonal trends in the occurrence of eclampsia. Natl Med J India. 1993;6(1):17–18.
- Bergström S, Povey G, Songane F, et al. Seasonal incidence of eclampsia and its relationship to meteorological data in mozambique. J Perinat Med. 1992;20(2):153–158. doi: 10.1515/jpme.1992.20.2.153
- Alderman BW, Boyko EJ, Loy GL, et al. Weather and occurrence of eclampsia. Int J Epidemiol. 1988;17(3):582–588.
- Wu S, Deng F, Huang J, et al. Does ambient temperature interact with air pollution to alter blood pressure? A repeated-measure study in healthy adults. J Hypertens. 2015;33(12):2414–2421.
- Cabrera SE, Mindell JS, Toledo M, et al. Associations of blood pressure with geographical latitude, solar radiation, and ambient temperature: results from the Chilean Health Survey, 2009–2010: table 1. Am J Epidemiol. 2016;183(11):1071–1073. doi: 10.1093/aje/kww037
- Sun S, Weinberger KR, Spangler KR, et al. Ambient temperature and preterm birth: a retrospective study of 32 million US singleton births. Environ Int. 2019;126:7–13. doi: 10.1016/j.envint.2019.02.023