ABSTRACT
Objective
SARS-CoV-2 infection during pregnancy has been linked with an increased risk of hypertensive disorders of pregnancy (HDP). The aim of this study was to examine how both trimester and severity of SARS-CoV-2 infection impact HDP.
Methods
We conducted a cohort study of SARS-CoV-2-infected individuals during pregnancy (n = 205) and examined the association between trimester and severity of infection with incidence of HDP using modified Poisson regression models to calculate risk ratios (RR) and 95% confidence intervals (CI). We stratified the analysis of trimester by severity to understand the role of timing of infection among those with similar symptomatology and also examined timing of infection as a continuous variable.
Results
Compared to a reference cohort from 2018, SARS-CoV-2 infection did not largely increase the risk of HDP (RR: 1.17; CI:0.90, 1.51), but a non-statistically significant higher risk of preeclampsia was observed (RR: 1.33; CI:0.89, 1.98), in our small sample. Among the SARS-CoV-2 cohort, severity was linked with risk of HDP, with infections requiring hospitalization increasing the risk of HDP compared to asymptomatic/mild infections. Trimester of infection was not associated with risk of HDP, but a slight decline in the risk of HDP was observed with later gestational week of infection. Among patients with asymptomatic or mild symptoms, SARS-CoV-2 in the first trimester conferred a higher risk of HDP compared to the third trimester (RR: 1.70; CI:0.77, 3.77), although estimates were imprecise.
Conclusion
SARS-CoV-2 infection in early pregnancy may increase the risk of HDP compared to infection later in pregnancy.
Introduction
SARS-CoV-2 (COVID-19) infection during pregnancy is of concern due to increased risks of adverse maternal and neonatal outcomes. Early case series suggested an increased incidence of preeclampsia among pregnant patients with SARS-CoV-2 infection (Citation1). This finding has subsequently been observed in more rigorous single-center and multi-site cohort studies (Citation2–9). In addition to the growing body of literature showing an increased risk of preeclampsia associated with SARS-CoV-2 infection, differing risks based on symptom severity have also been noted (Citation10,Citation11). A meta-analysis demonstrated a four-fold increase in the risk of preeclampsia for severe compared to mild infection (Citation12). Most of the existing studies are limited to patients in their later stages of pregnancy or focus on SARS-CoV-2 infection upon delivery admission, thereby providing little information specifically about early pregnancy infection (Citation2–4,Citation8,Citation13–18). One study reported a slightly higher rate of HDP among pregnant patients with infection prior to 32 weeks gestation compared to those with later infection, while a large cohort study reported a higher rate of HDP with preterm delivery associated with first and second trimester infection compared to an uninfected reference group (Citation14,Citation19).
The biological mechanisms that link SARS-CoV-2 infection during pregnancy and preeclampsia are unknown but proposed pathways include direct endothelial injury via the “cytokine storm,” an interruption of normal placentation leading to malperfusion, and persistent placental infection leading to placental damage (Citation20–22). A “preeclampsia-like” syndrome was described among pregnant patients with severe SARS-CoV-2 infection in the third trimester, with the authors suggesting that this syndrome was a distinct entity and different from actual preeclampsia because the former was not associated with pathognomonic biomarkers of preeclampsia (Citation23). Several other studies have explored whether severe SARS-CoV-2 infection is associated with preeclampsia or mimics preeclampsia symptoms (Citation23–25). Examinations of the placentas of pregnancies with SARS-CoV-2 infection have identified pathologic patterns also observed among patients with HDP including thrombi in fetal vessels, decidual arteriopathy, and maternal vascular malperfusion (Citation21,Citation26,Citation27). Such pathologic change does not appear to depend on disease severity as it has been seen in placentas of pregnancies with asymptomatic infection (Citation26).
If SARS-CoV-2 infection impacts placental perfusion in a pattern that mirrors that found in preeclampsia, then infection occurring in closer proximity to placental development could relay greater risk for HDP, the overlap of syndromes notwithstanding. Using a prospective cohort of patients with SARS-CoV-2 infection during pregnancy, we sought to identify whether there was an increased risk of HDP depending on trimester and severity of SARS-CoV-2 infection.
Materials and methods
We conducted a prospective cohort study of 224 pregnant patients with confirmed SARS-CoV-2 infection. We included pregnant patients with a positive SARS-CoV-2 molecular test result from March through December 2020, who planned to deliver at Boston Medical Center. Boston Medical Center is the largest safety-net hospital in New England, caring for a large minority patient population primarily insured by Medicaid. We developed a data abstraction tool to collect information on demographic and reproductive characteristics, timing and symptoms of SARS-CoV-2 infection, and delivery and birth outcomes from medical records. We excluded patients with a pregnancy loss prior to 20 weeks’ gestation (n = 8) and patients who did not deliver at the hospital (n = 11). We used the Clinical Data Warehouse at Boston University Medical Center to extract data on all 2018 deliveries at the same hospital to serve as an uninfected comparison cohort, thereby eliminating the possibility of undetected SARS-CoV-2 infection during any trimester in the unexposed group. This study was approved by the Boston University Medical Campus IRB (H-40400). A waiver of consent was obtained as this is a data repository study. The data that support the findings of this study are available upon request from author (V.S.) upon approval from our IRB.
Our primary exposure of interest was SARS-CoV-2 infection during pregnancy. We defined SARS-CoV-2 infection as a positive molecular test while pregnant. We further examined SARS-CoV-2 infection by trimester and symptoms. Gestational age at the time of symptom start, among symptomatic patients, and at the time of first positive molecular test, for all included patients, was calculated based on timing relative to the pregnancy start date (calculated as delivery date minus gestational age at delivery). During the study period, SARS-CoV-2 testing was conducted among patients with known exposures or symptoms and beginning on 27 April 2020, universal testing at delivery admission to labor and delivery regardless of symptoms. All testing was done by nasopharyngeal polymerase chain reaction (PCR) sampling. All patients with positive results were asked a standard questionnaire that touched on symptoms, sick contacts, and housing context. This information was documented in the electronic medical record and updated until 14 days after illness identification. Data on specific symptoms including fever, cough, shortness of breath, myalgia, anosmia, headache, sore throat, and gastrointestinal symptoms were abstracted from patient records through review of all notes including telephone, telemedicine, and inpatient and outpatient visit notes. To best align with previously used illness severity classifications, we defined illness as asymptomatic, mild (any combination of symptoms excluding shortness of breath), moderate (shortness of breath), and severe (requiring hospitalization) (Citation13).
The outcome of interest was HDP: gestational hypertension, preeclampsia, superimposed preeclampsia, eclampsia, and HELLP syndrome. All diagnoses from the patient problem list, in addition to blood pressure values and relevant laboratory results, during pregnancy and the delivery hospitalization were abstracted to classify HDP in accordance with guidelines from the American College of Obstetrics and Gynecology (Citation28). Postpartum hypertension and preeclampsia was not included as part of the outcome definition. Medical records were reviewed by a maternal-fetal medicine specialist (C.D.Y) to confirm diagnoses. We also calculated the gestational age at the onset of HDP. Pregnant patients with diagnoses of HDP prior to SARS-CoV-2 infection (n = 4) were not included in the calculation of associational measures.
We examined the distribution of maternal demographic and reproductive characteristics among patients with SARS-CoV-2 infection and the unexposed cohort of patients delivering in 2018. Among those with SARS-CoV-2 infection, we also examined the distribution by trimester of infection and severity of symptoms. We collected data on the following covariates, maternal age at delivery (categorized as ≤ 20; 21–25; 26–30; 31–35; ≥36), self-reported maternal race/ethnicity (white, non-Hispanic; Black, non- Hispanic; Hispanic; Asian, other/unknown), gravidity, parity, insurance (private, public, or other), and selected chronic conditions based on ICD-10 codes, including pre-pregnancy chronic hypertension, type 1 and type 2 diabetes, and asthma. We created a dichotomous comorbidity variable to indicate the presence of preexisting hypertension, diabetes, or asthma. Pre-pregnancy body mass index (BMI) was abstracted when available, otherwise the earliest pregnancy BMI measurement prior to 20 weeks’ gestation was used.
To examine the association between SARS-CoV-2 infection and any HDP, as well as its sub-types: gestational hypertension and preeclampsia, including eclampsia, superimposed, and HELLP, we used modified Poisson regression models to calculate risk ratios (RR) and 95% confidence intervals (CI) using the unexposed cohort as the reference group (Citation29). We calculated RRs adjusting for selected confounders reported in the literature including maternal age and comorbidity indicator (Citation13) and conducted analyses stratified by race/ethnicity (Black, White, Hispanic). To assess the impact of trimester of infection and severity of symptoms on the incidence of HDP among patients with COVID-19 infection, we calculated RRs for trimester, using third trimester infection as the reference, and severity, using asymptomatic and mild infections as a combined reference. We also explored timing of infection during pregnancy as a continuous variable, operationalized as the gestational age at the timing of symptom onset or if asymptomatic, the timing of the first positive test. We used restricted cubic splines with three knots to examine nonlinearity of the relationship between gestational week of infection and risk of HDP (Citation30). Finally, we stratified the analysis of trimester by severity (asymptomatic/mild and moderate/severe) to understand the impact of timing of infection among patients presenting with similar symptomatology. We performed additional sensitivity analyses excluding patients with chronic hypertension and then patients with any comorbidity to examine the association between SARS-CoV-2 infection and HDP among those with lower risk of both severe complications from SARS-CoV-2 and HDP. We also conducted a sensitivity analysis excluding cases detected prior to the onset of universal testing on 27 April 2020. Given the potential for small sample sizes in selected strata, the exploratory nature of our research question, and guidelines from the American Statistical Association, we do not rely solely on statistical significance (p < 0.05) for inference (Citation31). Instead, we present the risk ratios and 95% confidence to guide interpretation of the estimates with regard to magnitude, direction, and precision.
Results
We included 205 pregnant individuals with SARS-CoV-2 infection between March and December 2020 and with corresponding delivery dates from March 2020 through July 2021. Compared to the unexposed cohort, patients in the SARS-CoV-2 cohort were more likely to be younger than 25 years (30.7% vs. 22.8%), report Hispanic ethnicity (64.4% vs. 37.0%), and be publicly insured (84.9% vs. 77.9%). Pregnant patients with SARS-CoV-2 were also more likely to be obese and have chronic conditions, including hypertension and asthma. There was little difference in the rate of preterm birth (13.2% versus 10.8%; ).
Table 1. Maternal and reproductive characteristics of SARS-CoV-2 cohort and unexposed cohort, boston medical center.
Among the SARS-CoV-2 cohort, the risk of any HDP was 23.4% (n = 48), which was slightly higher than the risk in the unexposed cohort (19.3%; adjusted RR: 1.17; 95% CI: 0.90, 1.51). Upon examination of specific HDP subtypes, the elevation in risk was driven by preeclampsia (RR: 1.33; 95% CI: 0.89, 1.98), not gestational hypertension. Adjustment for maternal age and comorbidity index (preexisting hypertension, diabetes, or asthma) did not greatly alter observed associations compared to crude measures (). Upon stratification by race/ethnicity, we observed similar risk ratios for HDP among Black, White, and Hispanic patients. Among Black patients, SARS-CoV-2 was associated with a notable increase in the risk of preeclampsia, specifically, while among Hispanic patients, similar risk ratios were observed regardless of HDP sub-type. These risk ratio estimates were non-statistically significant and numbers were too small to estimate risk ratios for specific HDP sub-types among white patients ().
Table 2. Crude and adjusted risk ratios and 95% confidence intervals for SARS-CoV-2 infection and hypertensive disorder of pregnancy overall and by subtype.
Table 3. Crude risk ratios and 95% confidence intervals for SARS-CoV-2 infection and hypertensive disorder of pregnancy overall and by subtype, by race/ethnicity.
In the SARS-CoV-2 cohort the majority of infections were in the third trimester (56.6%), followed by the second trimester (32.2%) and first trimester (11.2%). Of note, a quarter (n = 8) of all documented first trimester infections were excluded due to pregnancy loss. The mean interval between symptom onset and pregnancy loss was 29 days. The mean age of pregnancy loss was 10 weeks. Compared to third trimester infection, the risk of HDP was 9% and 30% higher for SARS-CoV-2 in the first or second trimester, respectively (). Using a restricted cubic spline to model the association between gestational week of infection and risk of HDP adjusted for comorbidities, the test for curvature was non-significant (p-value = 0.36). We therefore present the linear relationship between gestational age of infection and risk of HDP, using 20 weeks as the referent, in . As the gestational age of infection increases, the risk of HDP decreases, although the 95% confidence intervals were wide and the overall model was non-significant.
Figure 1. Risk ratios and 95% confidence intervals for gestational week of infection and hypertensive disorders of pregnancy, restricted cubic spline.
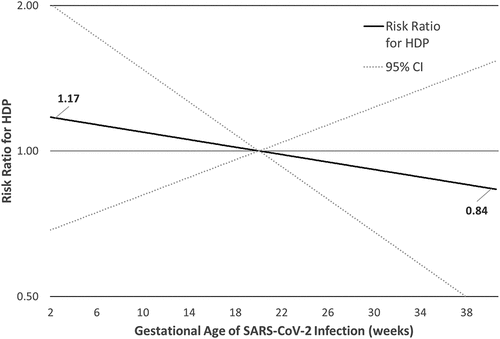
Table 4. Crude and adjusted risk ratios and 95% confidence intervals for SARS-CoV-2 infection and hypertensive disorders of pregnancy, by trimester and severity, SARS-CoV-2 cohort.
Twenty-four (11.8%) of the patients in our cohort had severe SARS-CoV-2 infection, defined as requiring hospitalization – six of whom were admitted to the intensive care unit. Mild and moderate infections were present in 59.3% and 14.7% of patients, respectively. Finally, just 14.2% of patients were asymptomatic. Due to the small number of cases in the asymptomatic group, we combined those with asymptomatic and mild infection to serve as the reference group. Compared to this referent, those with moderate infection and severe infections experienced 40% and 74% increases in the risk of HDP, respectively ().
We stratified the analysis of trimester of infection and risk of HDP by severity (asymptomatic/mild and moderate/severe). Among those with asymptomatic or mild disease, the risk of HDP in those with first trimester infection was 30.0% compared to 17.4% in those with third trimester infection (RR: 1.70; 95% CI: 0.77, 3.77). The sample size for moderate and severe infection was small, but notably the risk of HDP was 38% and 30% for second and third trimester, respectively, which was much higher than asymptomatic and mild infections during those time frames. ()
Table 5. Risk ratios and 95% confidence intervals for trimester of SARS-CoV-2 and hypertensive disorders of pregnancy, stratified by symptom severity, SARS-CoV-2 cohort.
In sensitivity analyses restricted to the lower-risk cohorts, the association between SARS-CoV-2 infection and HDP was attenuated (RR: 1.06, 95% CI: 0.80, 1.41 among those without chronic hypertension; RR: 1.10, 95% CI: 0.79, 1.52 among those without a comorbidity). The association between SARS-CoV-2 and HDP was also attenuated upon the removal of cases prior to 27 April 2020; RR: 1.10 (95% CI: 0.78, 1.55).
Discussion
We report a minimal increase in the risk of HDP associated with SARS-CoV-2 infection during pregnancy compared to an unexposed cohort. The increase was driven by a higher risk of preeclampsia, not gestational hypertension, when compared to background rates in a historical cohort of deliveries prior to the SARS-CoV-2 pandemic, although this finding was non-statistically significant. These findings, suggestive of an increased risk, are consistent with a single-center cohort study that showed an increase in severe preeclampsia among the SARS-CoV-2 positive group, but not gestational hypertension or preeclampsia without severe features (Citation14). Our observation of a trend between increasing infection severity and increased risk of HDP is also consistent with numerous previously published studies (Citation12).
Our study also examined the impact that timing of infection has on the risk of HDP. Overall, we report a decline in the risk of HDP with increasing gestational age at first infection. The increased risk of HDP associated with first trimester infection compared to third trimester infection was most notable among patients with asymptomatic and mild infection, yet this finding was based on just six cases and the 95% confidence interval included the null. We were unable to examine this among patients with moderate and severe infections due to the limited sample size. In our cohort, 26% of our pregnant patients with first trimester infection suffered spontaneous abortion on average 29 days after symptom onset. A link between the COVID pandemic and increased risk of miscarriage has previously been noted (Citation32). The majority of losses occurred beyond 6 weeks gestational age when the loss rate should be less than 5% (Citation33).
Few studies have examined the explicit impact of COVID infection in the first trimester. A large systematic review and meta-analysis identified increased COVID-19 vaccine hesitancy in the first trimester compared to second or third, a finding at odds with the recommendation to get vaccinated and boosted at the earliest available time including periconception or during fertility treatment (Citation34). While infection in earlier pregnancy has been linked with some adverse perinatal outcomes, studies examining timing of infection and HDP have been less consistent. A large retrospective cohort of pregnancies in the first year of the COVID pandemic compared preeclampsia incidence in a population with third trimester exposure and a grouped population of first or second trimester exposure and observed no difference in the risk of HDP with different timepoints of infection across pregnancy (Citation35). Neelam et al. investigated gestational hypertension across trimesters of infection and found no difference but did not separate gestational hypertension from preeclampsia- a relevant distinction that underscored a difference in our analysis (Citation36). Additionally, both studies had patient populations that were predominantly white with smaller representation of racial and ethnic groups that are disproportionately affected by both COVID and preeclampsia. A recently published large cohort study reported an increased risk of HDP with delivery at <37 weeks of gestation associated with infection prior to 28 weeks gestation using an uninfected comparison group (Citation19).
The basic model of preeclampsia pathophysiology is that early placental malperfusion leads to the release of anti-angiogenic and inflammatory mediators that impart endothelial dysfunction (Citation37,Citation38). The same defects in trophoblast invasion and uterine spiral arterial remodeling are also inextricably linked to early fetal support. Indeed, a recent study found 32.5% of placentas of patients with COVID infection showed fetal vascular malperfusion (Citation26). They focused on a cohort of pregnant people who delivered in the early months of the pandemic, thus almost all with late trimester exposure; therefore, there was no opportunity to study individuals exposed in the first trimester. Clarification of the role of COVID-19 infection in this pathway is critical to understanding whether interventions that target early vascularization of the placental bed such as aspirin may be effective in protecting pregnant people who suffer early infection (Citation39).
An important finding in our data was the high rate of HDP in our unexposed cohort. The rate of HDP was 19.3% in our historical cohort, which is higher than the national rate of 14.6% reported for the same calendar years by the Centers for Disease Control and Prevention (Citation40). The high rate of HDP in our patient population overall, both before and during the COVID pandemic, may be partially explained by the high proportion of historically marginalized patients and patients with comorbidities in our sample.
A strength of this study is the inclusion of over 200 pregnancies with PCR confirmed SARS-CoV-2 infection at any point during pregnancy. We were also able to use deliveries at the same institution from 2018 to serve as a comparison. While this was a historical comparison, it was prior to the first documented cases of COVID-19 in the United States and therefore would not include any pregnancies with undiagnosed infections. We abstracted data from medical records including visit summaries, progress notes, and laboratory values, which enhanced data quality and completion. Furthermore, we were able to collect detailed symptom data to classify infection severity. We used published symptom severity classifications to guide our symptom categories and were able to align our mild and moderate categories closely (Citation13). We instituted measures to ensure data quality including abstraction training, dual reviewer abstractions, and comprehensive outcome review for classification by a maternal-fetal medicine specialist. Another strength is the availability of symptom start date, first positive test date and HDP diagnosis date, allowing for the establishment of temporality. This has been a limitation of many studies that have used SARS-CoV-2 results from universal screening at delivery admission (Citation2,Citation18).
While this study constitutes a rich cohort of pregnant patients with SARS-CoV-2 infections, the sample size, particularly for first trimester infections, was small. This led to imprecise estimates and limited our ability to adjust for additional covariates. Additionally, as noted above, the loss of pregnancies in the early first trimester may be related to vascular pathology that would otherwise have contributed to HDP. Furthermore, our cohort included pregnant patients from March through December 2020, which reflects the early stages of the pandemic and includes some time prior to implementation of universal screening at admission to labor and delivery and prior to widely available testing. Our cohort, like others, may over represent severe infections. Due to the use of a historical and uninfected comparison group that has a different demographic profile, the two groups may not be entirely comparable in assessing risk of HDP solely based on the presence or absence of COVID infection and therefore we cannot rule out the possibility of confounding, particularly due to factors such as temporal trends in HDP and pandemic related stress. We adjusted for chronic hypertension, diabetes, and asthma – common chronic conditions known to increase COVID severity and risk of HDP – in order to reduce confounding by comorbidities (Citation41), but did not adjust for early pregnancy BMI due to the high proportion of missing data. Sensitivity analyses restricted to patients without comorbidities and restricted to cases occurring after universal testing implementation reduced the magnitude of estimates suggesting effect measure modification. In the analyses of timing and severity, which were restricted to the SARS-CoV-2 cohort, confounding by calendar time and changes in disease dynamics, including transmission, testing, treatment, and strains, is possible and may also render results less generalizable to subsequent time periods.
Our findings were based on physiologic effects of the variants present in the first year of the COVID pandemic at a single safety-net institution. These trends should be investigated further within cohorts with detailed data on timing of both COVID infection and HDP diagnosis. Clarifying the vascular impact of early COVID infection may help support public health campaigns that recommend early vaccination and boosting in pregnancy.
Highlights
SARS-CoV-2 infection in pregnancy did not largely increase the risk of hypertensive disorders of pregnancy.
SARS-CoV-2 infection with severe symptoms was associated with a higher risk of hypertensive disorders of pregnancy compared to infection with mild symptoms.
As gestational week at the time of infection increased a slight decline in the risk of hypertensive disorders of pregnancy was observed.
Acknowledgments
We are grateful to the BUSPH Epidemiology COVID-19 Response Corps for creating a network that catalyzed the formation of the team that conducted this epidemiologic research on COVID-19.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. doi: 10.1016/j.ajogmf.2020.100107
- Cardona-Perez JA, Villegas-Mota I, Helguera-Repetto AC, et al. Prevalence, clinical features, and outcomes of SARS-CoV-2 infection in pregnant women with or without mild/moderate symptoms: results from universal screening in a tertiary care center in Mexico city, Mexico. PloS One. 2021;16(4):e0249584. doi: 10.1371/journal.pone.0249584
- Abedzadeh-Kalahroudi M, Sehat M, Vahedpour Z, et al. Maternal and neonatal outcomes of pregnant patients with COVID-19: a prospective cohort study. Int J Gynaecol Obstet. 2021;153(3):449–9. doi: 10.1002/ijgo.13661
- Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782–5. doi: 10.1001/jama.2020.19124
- Barbero P, Muguerza L, Herraiz I, et al. SARS-CoV-2 in pregnancy: characteristics and outcomes of hospitalized and non-hospitalized women due to COVID-19. J Matern Fetal Neonatal Med. 2020;35(14):2648–2654. doi: 10.1080/14767058.2020.1793320
- Brandt JS, Hill J, Reddy A, et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol. 2021;224(4):.e389.1–.e389.9. doi: 10.1016/j.ajog.2020.09.043
- Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817. doi: 10.1001/jamapediatrics.2021.1050
- Jering KS, Claggett BL, Cunningham JW, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181(5):714–7. doi: 10.1001/jamainternmed.2020.9241
- Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021 Sep 1;225(3):289–e1. doi: 10.1016/j.ajog.2021.05.014
- Delahoy MJ, Whitaker M, O’Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 — COVID-NET, 13 states, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1347–1354. doi: 10.15585/mmwr.mm6938e1
- Lai J, Romero R, Tarca AL, et al. SARS-COV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose response relationship supporting causality. Am J Obstet Gynecol. 2021;225(6):689–693.e1. doi: 10.1016/j.ajog.2021.08.020
- Wei SQ, Bilodeau-Bertrand M, Liu S, et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193(16):E540–E548. doi: 10.1503/cmaj.202604
- Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137(4):571–80. doi: 10.1097/AOG.0000000000004339
- Rosenbloom JI, Raghuraman N, Carter EB, et al. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2021;224(6):623–4. doi: 10.1016/j.ajog.2021.03.001
- Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3(11):e2029256. doi: 10.1001/jamanetworkopen.2020.29256
- Zhang L, Dong L, Ming L, et al. Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China. Bmc Pregnancy Childbirth. 2020;20(1):394. doi: 10.1186/s12884-020-03026-3
- Gurol-Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225(5):.e522.1–.e522.11. doi: 10.1016/j.ajog.2021.05.016
- Palmsten K, Vazquez-Benitez G, Kharbanda EO. Point: uncertainty about estimating the risks of COVID-19 during pregnancy. Paediatric Perinatal Epid. 2021;36(4):450–452. doi: 10.1111/ppe.12773
- Hughes BL, Sandoval GJ, Metz TD, et al. First- or second-trimester SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol. 2023 Feb;228(2):.e226.1–.e226.9. do i:
- Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–87. doi: 10.1093/cvr/cvaa106
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089
- Fabre M, Calvo P, Ruiz-Martinez S, et al. Frequent placental SARS-CoV-2 in patients with COVID-19-associated hypertensive disorders of pregnancy. Fetal Diagn Ther. 2021;48(11–12):801–811. doi: 10.1159/000520179
- Mendoza M, Garcia-Ruiz I, Maiz N, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127(11):1374–80. doi: 10.1111/1471-0528.16339
- Rolnik DL. Can COVID-19 in pregnancy cause pre-eclampsia? BJOG. 2020;127(11):1381. doi: 10.1111/1471-0528.16369
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0
- Patberg ET, Adams T, Rekawek P, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224(4):.e382.1–.e382.18. doi: 10.1016/j.ajog.2020.10.020
- Baergen RN, Heller DS. Placental pathology in COVID-19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23(3):177–80. doi: 10.1177/1093526620925569
- Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135(6):e237–e260. doi: 10.1097/AOG.0000000000003891
- Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. doi: 10.1002/sim.4780080504
- Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat. 2016;70(2):129–133. doi: 10.1080/00031305.2016.1154108
- Sacinti KG, Kalafat E, Sukur YE, et al. Increased incidence of first‐trimester miscarriage during the COVID‐19 pandemic. Ultrasound Obstet Gynecol. 2021;57(6):1013. doi: 10.1002/uog.23655
- Tong S, Kaur A, Walker SP, et al. Miscarriage risk for asymptomatic women after a normal first-trimester prenatal visit. Obstet Gynecol. 2008;111(3):710–4. doi: 10.1097/AOG.0b013e318163747c
- Bianchi FP, Stefanizzi P, Di Gioia MC, et al. COVID-19 vaccination hesitancy in pregnant and breastfeeding women and strategies to increase vaccination compliance: a systematic review and meta-analysis. Expert Rev Vaccines. 2022;21(10):1443–54. doi: 10.1080/14760584.2022.2100766
- Getahun D, Peltier MR, Lurvey LD, et al. Association between SARS-CoV-2 infection and adverse perinatal outcomes in a large health maintenance organization. Am J Perinatol. 2022 Jun;41(2):199–207. doi: 10.1055/s-0042-1749666
- Neelam V, Reeves EL, Woodworth KR, et al. Pregnancy and infant outcomes by trimester of SARS-CoV -2 infection in pregnancy– SET-NET , 22 jurisdictions, January 25, 2020–December 31, 2020. Birth Defects Res. 115(2):145–159. doi: 10.1002/bdr2.2081
- Burton GJ, Redman CW, Roberts JM, et al. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381
- Narang K, Enninga EA, Gunaratne MD, et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. InMayo Clinic Proceedings. 2020 Aug 1;95(8):1750–1765. doi: 10.1016/j.mayocp.2020.05.011
- Dutta S, Kumar S, Hyett J, et al. Molecular targets of aspirin and prevention of preeclampsia and their potential association with circulating extracellular vesicles during pregnancy. Int J Mol Sci. 2019;20(18):4370. doi: 10.3390/ijms20184370
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization—United States, 2017–2019. Morbidity Mortality Weekly Rep. 2022;71(17):585. doi: 10.15585/mmwr.mm7117a1
- Smith ER, Oakley E, Grandner GW, et al. Clinical risk factors of adverse outcomes among women with COVID-19 in the pregnancy and postpartum period: a sequential, prospective meta-analysis. Am J Obstet Gynecol. 2022;228(2):161–177. doi: 10.1016/j.ajog.2022.08.038

