?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
The objective was to utilize a smartwatch sphygmomanometer to predict new-onset hypertension within a short-term follow-up among individuals with high-normal blood pressure (HNBP).
Methods
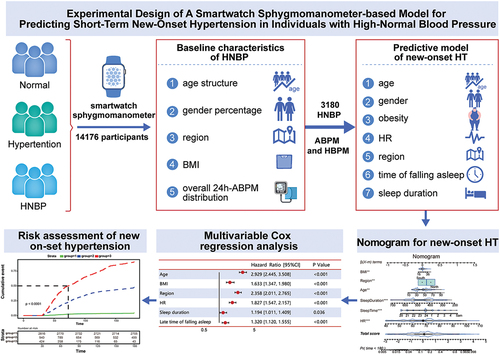
This study consisted of 3180 participants in the training set and 1000 participants in the validation set. Participants underwent both ambulatory blood pressure monitoring (ABPM) and home blood pressure monitoring (HBPM) using a smartwatch sphygmomanometer. Multivariable Cox regressions were used to analyze cumulative events. A nomogram was constructed to predict new-onset hypertension. Discrimination and calibration were assessed using the C-index and calibration curve, respectively.
Results
Among the 3180 individuals with HNBP in the training set, 693 (21.8%) developed new-onset hypertension within a 6-month period. The nomogram for predicting new-onset hypertension had a C-index of 0.854 (95% CI, 0.843–0.867). The calibration curve demonstrated good agreement between the nomogram’s predicted probabilities and actual observations for short-term new-onset hypertension. In the validate dataset, during the 6-month follow-up, the nomogram had a good C-index of 0.917 (95% CI, 0.904–0.930) and a good calibration curve. As the score increased, the risk of new-onset hypertension significantly increased, with an HR of 8.415 (95% CI: 5.153–13.744, p = .000) for the middle-score vs. low-score groups and 86.824 (95% CI: 55.071–136.885, p = .000) for the high-score vs. low-score group.
Conclusions
This study provides evidence for the use of smartwatch sphygmomanometer to monitor blood pressure in individuals at high risk of developing new-onset hypertension in the near future.
Trial registration
ChiCTR2200057354
Introduction
High-normal blood pressure (HNBP) is between a normal BP value and hypertension,Citation1 and its definition is currently controversial. The 2022 Chinese Hypertension Guideline published a new HNBP criterion of an SBP of 120–129 mmHg and a DBP less than 80 mmHg,Citation2 which is consistent with the 2017 ACC/AHA guidelinesCitation3 and differs from the 2018 ESC/ESH Guidelines.Citation4 The incidence of HNBP varies significantly due to different diagnostic criteria. In China, the incidence of HNBP is 36.4%, with 41.1% of cases occurring in males and 33.2% of cases occurring in femalesCitation5. Previous studies have shown that the risk of new-onset hypertension in HNBP populations within a 4-year period can be as high as 51.7%Citation6, and HNBP is frequently associated with different metabolic abnormalities that increase an individual’s cardiovascular (CV) riskCitation7. Unfortunately, HNBP has not received due attention because there are no obvious clinical symptoms. Previous studies recommended lifestyle adjustment and long-term follow-up for HNBP. The lack of targeted advice and long-term follow-up of all HNBP populations will require massive financial and medical resources but provide low health economic benefits. Therefore, it is crucial to identify individuals with HNBP at high risk of new-onset hypertension early and provide comprehensive management.Citation8
However, there is a lack of effective predictive models for new-onset hypertension in individuals with HNBP due to unreliable questionnaire and retrospective self-measured blood pressure records and poor compliance. Recently, mobile, and wearable devices, such as smartphones, smartwatches, and tablet PCs, have improved medical management, including BP management.Citation9 In this study, we used physiological parameters monitored by a smartwatch to establish a model for the prediction of short-term new-onset hypertension in people with HNBP and stratify the risk of people with HNBP to facilitate hierarchical management to achieve effective control. This study provides innovative evidence for the use of smartwatch sphygmomanometer and app applications to manage HNBP populations at high risk of new-onset hypertension in the short term.
Methods
This study was approved by the institutional ethics committee of Chinese PLA General Hospital (ChiCTR2200057354). The electronic informed consent of individuals in the validation group was obtained before enrollment. We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines for this cohort study.
This was a longitudinal cohort study. From among 14 176 participants, there were 3,180 individuals with HNBP who met the inclusion criteria and had undergone both ambulatory blood pressure monitoring (ABPM) and home blood pressure monitoring (HBPM) for at least 6 months with a smartwatch sphygmomanometer before enrollment, which was used for predictive model. For the validate set, we prospectively enrolled 1000 individuals from the cohort of the other HNBP participants who voluntarily participated in this study and signed electronic informed consent forms in September 2022, and underwent ABPM and HBPM, followed by a 6-month follow-up. The inclusion criteria were as follows: (1) aged 18 years or older; (2) completed a baseline questionnaire online, included smoke, alcohol use, body mass index (BMI), age, sex, and residential area; (3) recorded HR, time of falling asleep and sleep duration data; (4) diagnosed with HNBP with ABPM between January and March 2022; and (5) could implement HBPM in the next 6 months. The exclusion criteria were as follows: (1) normal BP; (2) hypertension or a history of hypertension; (3) anti-hypertensive treatment; (4) failure to complete measurements of ABPM, HBPM, HR and sleep parameters.
The risk of developing hypertension within the next 4 years for individuals with HNBP was 51.7%Citation6. The estimated risk of new-onset hypertension within 6 months was calculated to be 8.7% using the formula (P0.5 = 1 = 1
) = 0.087), 140/8.7% = 1609. The minimum sample size for the prediction model was 1609 individuals. The validation set followed the events per variable (EPV) principle, and the minimum number required was 140 (20
7 = 140). The validation set for this study comprised1000 individuals.
We utilized a smartwatch sphygmomanometer (Huawei Watch D) registered as Class II medical device in China (NMPA: 20212071428) and with Conformité Européene certification (CE: HZ 2 042 319–1) in accordance with ISO 81 060–2:2018 standards.Citation10,Citation11 According to the 2021 ESH guidelinesCitation12, ABPM was used for the initial enrollment period, and HBPM was used for next follow-up. The ABPM frequency was set at every 20 minutes during the daytime and every 30 minutes at night. A number of readings exceeding 20 during the daytime and 7 at night was considered valid.Citation12 HNBP was defined as an average 24-hour ABPM systolic blood pressure (SBP) between 120 and 129 mmHg or an average diastolic blood pressure (DBP) between 75 and 79 mmHg. Both ABPM and HBPM were measured by a smartwatch sphygmomanometer (Huawei Watch D) through an oscillographic method.
For ABPM measurements, automatic measurements are taken every 30 minutes in the awake state, and the subjects are required to complete the measurements according to the standard posture. Automatic measurements are taken every 45 minutes while the subjects are asleep, and there is no need for subjects to cooperate in adjusting their posture. The sleep detection of smart wearable devices automatically recognizes the subjects’ sleep status and intelligently switches to automatic BP measurement mode at night. The smart sphygmomanometer uses HUAWEI TruSleepTM sleep monitoring technology to extract sinus rhythm interval sequences and respiratory signals from PPG signals. Through Hilbert – Huang (HHT) transformation technology, sleep conditions can be accurately analyzed, and nighttime BP measurements can be automatically initiated.
Follow-up was conducted using a mobile app. All enrolled individuals completed HBPM for 7 days per month (at least 3 days with at least 12 readings) during the next 6 months. Online training was provided to the individuals, and BP was measured in a quiet environment after a rest of 3 to 5 minutes. Measurements were taken at intervals of 1 to 2 minutes, with at least 5 consecutive days of monitoring. If the difference between the first and second readings exceeded 10 mmHg, a third measurement was recommended, and the average value of the latter two readings was used.Citation3
All enrolled individuals underwent HBPM through a smartwatch sphygmomanometer to identify new-onset hypertension. For newly identified hypertensive subjects, it is recommended to reverify BP with a medical upper arm or medical ambulatory sphygmomanometer at the outpatient clinic or with a home upper arm sphygmomanometer. The definition of new-onset hypertension referred to the hypertension standards for office BP, ABPM, or HBPM in the 2018 ESC/ESH Guidelines. New-onset hypertension was defined as an office BP exceeding 140/90 mmHg, an average BP exceeding 130/80 mmHg measured by ABPM, or an average BP exceeding 135/85 mmHg measured by HBPM.Citation4 If the diagnosis was based on office BP or ABPM from the hospital, medical records were uploaded.
Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. X-tile (v3.6) was used to determine the cutoff value of the risk scoreCitation13. The relationship between the continuous independent variables and new-onset hypertension was analyzed using a restricted cubic spline (RCS) diagram. Cox regression analysis was utilized for multivariate analyses. A significance level of p < .05 was used to determine statistical significance. A nomogram was created based on the results of multivariate Cox analysis and by using the rms packageCitation14 in R version 4.1.3 (The R Project for Statistical Computing, Vienna, Austria). The performance of the nomogram was assessed using the concordance index (C-index) and calibration curve. Bootstraps with 1,000 resample were utilized for these activities. For the validation of the nomogram, the total points for each patient in the validation set were calculated based on the established nomogram. The C-index and calibration curve were derived from the regression analysis. Finally, the whole dataset was used to construct a dynamic nomogram using the DynNom package for visualization of predictive models (Central Illustration).
Results
In the training set, 3,180 participants were included (), of whom 693 (21.8%) developed new-onset HT during the 6-month follow-up. Compared with HNBP without progression group, the new-onset HT group had a significantly higher age, HR, and BMI. The proportion of patients who resided in the northern region, slept later and had a short sleep duration in the new-onset HT group was higher than that in the HNBP without progression group (). There was no significant difference in smoke, alcohol use between two groups. The baseline characteristics of individuals with HNBP in the validation set are provided in .
Table 1. Baseline characteristics between training and validation dataset.
The results of the COX regression analysis are shown in . Multivariate COX regression revealed the following HRs: 1.046 (95% CI 0.854–1.280) for sex, 2.927 (95% CI 2.444–3.506) for age, 0.954 (95% CI 0.749, 1.216) for smoke, 0.893 (95% CI 0.690, 1.155) for alcohol use, 1.633 (95% CI 1.347–1.981) for BMI, 2.358 (95% CI 2.011–2.765) for residential region, 1.827 (95% CI 1.547–2.157) for heart rate, 1.193 (95% CI 1.011–1.408) for sleep duration and 1.319 (95% CI 1.119–1.554) for time of falling asleep.
Table 2. COX regression analysis of the training dataset*
The nomogram that incorporated all significant independent factors for short-term new-onset hypertension in the predictive dataset was shown in . The C-index for predicting short-term new-onset hypertension was 0.854 (95% CI, 0.843, 0.867). An RCS diagram demonstrated the relationship between continuous variables and the risk of new-onset hypertension (). Additionally, the calibration plot demonstrated an agreement between the nomogram’s predicted probability of new-onset hypertension at 6-month and the actual observations ().
Figure 2. Nomogram for new-onset hypertension in HNBP populations. To use the nomogram, locate the individual’s value on each variable axis, and draw a line upward to determine the corresponding points. The sum of these points is located on the total points axis, and a line is drawn downwards to the accumulative axes to determine the likelihood of new-onset hypertension within 6 months.

Figure 3. Restricted cubic spline diagram. (a): RCS of age. (b): RCS of BMI. (c): RCS of HR. (d): RCS of sleep duration.

Figure 4. The calibration curve for predicting new-onset hypertension at 6-month in the training set and validation set. (a): the calibration curve for predicting new-onset hypertension at 6 months in the training set. (b): the calibration curve for predicting new-onset hypertension in 6-month in the validation set.

In the validation dataset, the incidence of new-onset hypertension within 6 months was 24.0%. The nomogram’s C-index for the short-term prediction of new-onset hypertension was 0.917 (95% CI, 0.904, 0.930). The calibration curve depicted good agreement between the predicted and observed probabilities of new-onset hypertension at the 6-month mark ().
A dynamic network nomogram was constructed (available at https://hnbp.shinyapps.io/DynNomapp) to visualize and predict the short-term risk of new-onset hypertension for individuals with different risk score of new-onset hypertension. At the 6-month follow-up, we compared the risk of new-onset hypertension among individuals with low (0–7.81), medium (7.82–9.93) and high (≥9.94) scores according to the nomogram. The high-score group exhibited the highest risk of new-onset hypertension compared to the low-score group (HR 86.824, 95% CI 55.071–136.885, p < .001). The medium-score group had a higher risk of new-onset hypertension than the low-score group (HR 8.415, 95% CI, 5.153–13.744, p < .001). The Kaplan Meier survival curve is shown in .
Discussion
In this study, HNBP was defined as an average 24-hour SBP between 120 and 129 mmHg or DBP between 75 and 79 mmHg measured by ABPM.Citation4 With this definition, this study suggests that approximately 20% of individuals with HNBP may progress to hypertension in the short term, highlighting the substantial disease burden associated with HNBP. In this study, the findings suggest that the risk of new-onset hypertension in individuals with HNBP is associated with age, BMI, HR, residence in Northern China, a short sleep duration, and later time of falling asleep. BMI, HR, sleep duration and time of falling asleep can be modified. In this study, 61.9% of individuals were overweight, and 13.1% were obese. Worldwide, over 600 million adults are obese, which is associated with an increased risk of all-cause deaths and cardiovascular diseases.Citation15 The prevalence of general obesity and abdominal obesity in China has increased significantly, and the mortality rates of overweight and obesity have doubled.Citation16,Citation17 Effective interventions targeting obesity will significantly reduce the incidence and mortality rates of cardiovascular diseases and hypertension.Citation18 This study also suggests that both a short sleep duration and a later time of falling asleep are important risk factors for the progression of HNBP to new-onset hypertension.
Previous research data on HNBP, such as sleep and exercise, were mostly from subject reviews, with slightly larger accuracy errors, and the heart rate was not monitored over a long period of time. In this study, smart watches are used to accurately record physiological data such as sleep and exercise and to continuously monitor heart rate over a long period of time, with high data accuracy and a more realistic physiological level. In this study, the physiological data monitored by the smart watch were used to establish a model, and the risk stratification of the population with HNBP could better guide medical staff in stratifying the management of the relevant population and providing different individuals with more targeted suggestions, such as improving sleep habits and weight loss, which improved compliance and had good health economic benefits. Additionally, it is also convenient for patients to carry out more targeted indicator monitoring and self-management according to their own conditions. Therefore, it is urgent to establish a new smart management mode for HNBP.Citation19,Citation20 Mobile medical devices can improve self-management for chronic diseases.Citation21,Citation22 Meta-analyses have demonstrated that a comprehensive management model could improve the control rate of hypertension.Citation23,Citation24 A study demonstrated that mHealth interventions can enhance cognition and improve the cardiometabolic profile of individuals with HNBP in low-resource urban settings.Citation25 In this study, the smartwatch sphygmomanometer collects real-time, long-term HBPM data and multiple parameters, including electronic and sleep parameters. By integrating these data with a mobile app, the smartwatch sphygmomanometer provided convenience for data uploading, storage, and analysis and managing large populations. This approach surpasses the conventional medical model by providing more accurate and comprehensive BP measurements. In addition, the smartwatch sphygmomanometer (Huawei Watch D) incorporates a daytime high BP alert function and BP circadian rhythm function, utilizing photoplethysmography (PPG) technology and an artificial intelligence (AI) algorithm. It delivers health education to individuals at high risk through mobile apps. This education encompasses advice on smoking cessation, weight management, dietary control, and exercise rehabilitation etc. To a certain extent, this aids hypertensive patients or individuals with HNBP to control their BP through lifestyle modifications. The HERB-DH1 study used smartphone applications for digital intervention treatment in hypertensive patients, effectively reducing blood pressure.Citation26
Not only is the definition of HNBP controversial, but there is also debate about how to intervene for people with this blood pressure level, whether medication is needed, and when medication should be started. However, for this group of people, especially high-risk individuals, attention should be given to strengthening follow-up and lifestyle guidance, such as improving weight loss and sleep habits, to control risk factors. This is the cornerstone of the management of people with HNBP.
In this study, we used a smartwatch sphygmomanometer to measure ABPM and HBPM through an oscillographic method, and its accuracy had been proven elsewhereCitation10,Citation11. Compared with other electronic sphygmomanometers, smartwatch sphygmomanometers offer real-time, long-term HBPM and ABPM measurements, as well as enable acquisition of multi-variable parameters. In this study, we innovatively developed an effective prediction model for short-term new-onset hypertension in the HNBP population utilizing a smartwatch sphygmomanometer. For example, for a high-risk participant with HNBP identified through this prediction model who had risk factors such as overweight, going to sleep late, and insufficient sleep duration, we can use mobile apps to send health education to help him control his diet, lose weight, adjust his sleep structure and regularly monitor BP to prevent new-onset hypertension. The model was validated and demonstrated good prediction and calibration. The results of this study will provide evidence for future digital interventions for HNBP populations at high risk of new-onset hypertension. In the future, the smartwatch sphygmomanometer could help more hypertensive patients or individuals with HNBP control their BP through lifestyle modifications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet. 2021;398(10304):957–8. doi:10.1016/S0140-6736(21)01330-1.
- Diseases NCfC, Association CMD, Committee CMDAH, cardiology CSo, Committee AomeatTsH. Practice guidelines of hypertension in China. Chinese J Cardiol. 2022, 50, 1050–95.
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–115. doi:10.1161/HYP.0000000000000065.
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. doi:10.1093/eurheartj/ehy339.
- Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18(11):785–802. doi:10.1038/s41569-021-00559-8.
- Julius S, Kaciroti N, Egan BM, Nesbitt S, Michelson EL. TROPHY study: outcomes based on the seventh report of the joint national committee on hypertension definition of hypertension. J Am Soc Hypertens. 2008;2(1):39–43. doi:10.1016/j.jash.2007.07.005.
- Mazza A, Schiavon L, Rigatelli G, Torin G, Lenti S. The effects of a new generation of nutraceutical compounds on lipid profile and glycaemia in subjects with pre-hypertension. High Blood Press Cardiovasc Prev. 2019;26(4):345–50. doi:10.1007/s40292-019-00332-6.
- Hanssen H, Boardman H, Deiseroth A, Moholdt T, Simonenko M, Kränkel N, Niebauer J, Tiberi M, Abreu A, Solberg EE, et al. Personalized exercise prescription in the prevention and treatment of arterial hypertension: a consensus document from the European Association of Preventive Cardiology (EAPC) and the ESC council on hypertension. Eur J Prev Cardiol. 2022;29(1):205–15. doi:10.1093/eurjpc/zwaa141.
- Krishnaswami A, Beavers C, Dorsch MP, Dodson JA, Masterson Creber R, Kitsiou S, Goyal P, Maurer MS, Wenger NK, Croy DS, et al. Gerotechnology for older adults with cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(22):2650–70. doi:10.1016/j.jacc.2020.09.606.
- Zhang W, Zhou YN, Zhou Y, Wang JG. Validation of the watch-type HUAWEI WATCH D oscillometric wrist blood pressure monitor in adult Chinese. Blood Press Monit. 2022;27(5):353–56. doi:10.1097/MBP.0000000000000608.
- Yi L, Lv ZH, Hu SY, Liu Y-Q, Yan J-B, Zhang H, Li H-B, Chen Q, Li Y-Y, Jiang Y-F, et al. Validating the accuracy of a multifunctional smartwatch sphygmomanometer to monitor blood pressure. J Geriatr Cardiol. 2022;19(11):843–52. doi:10.11909/j.issn.1671-5411.2022.11.004.
- Stergiou GS, Palatini P, Parati G, O’Brien E, Januszewicz A, Lurbe E, Persu A, Mancia G, Kreutz R. 2021 European society of hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–302. doi:10.1097/HJH.0000000000002843.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. doi:10.1158/1078-0432.CCR-04-0713.
- E. F, Jr. H. Rms: Regression Modeling Strategies. R Package version 3.4-0.
- Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH , Moradi-Lakeh M, Naghavi M, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377 (1): 13–27. doi:10.1056/NEJMoa1614362.
- Zhang X, Zhang M, Zhao Z, Huang Z, Deng Q, Li Y, Pan A, Li C, Chen Z, Zhou M, et al. Geographic variation in prevalence of adult obesity in China: results from the 2013–2014 national chronic disease and risk factor surveillance. Ann Intern Med. 2020;172(4):291–93. doi:10.7326/M19-0477.
- Collaborators GRF, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, Abdollahpour I. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1223–49. doi:10.1016/S0140-6736(20)30752-2.
- Roth GA, Nguyen G, Forouzanfar MH, Mokdad AH, Naghavi M, Murray CJ. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation. 2015;132(13):1270–82. doi:10.1161/CIRCULATIONAHA.115.016021.
- Allegrante JP, Wells MT, Peterson JC. Interventions to support behavioral self-management of chronic diseases. Annu Rev Public Health. 2019;40(1):127–46. doi:10.1146/annurev-publhealth-040218-044008.
- Reynolds R, Dennis S, Hasan I, Slewa J, Chen W, Tian D, Bobba S, Zwar N. A systematic review of chronic disease management interventions in primary care. BMC Fam Pract. 2018;19(1):11. doi:10.1186/s12875-017-0692-3.
- Alessa T, Hawley MS, Hock ES, de Witte L. Smartphone apps to support self-management of hypertension: review and content analysis. JMIR mHealth Uhealth. 2019;7(5):e13645. doi:10.2196/13645.
- Coughlin SS, Prochaska JJ, Williams LB, Besenyi G, Heboyan V, Goggans S, Yoo W, De Leo G. Patient web portals, disease management, and primary prevention. Risk Manag Healthc Policy. 2017;10:33–40. doi:10.2147/RMHP.S130431.
- Xiong S, Berkhouse H, Schooler M, Pu W, Sun A, Gong E, Yan LL. Effectiveness of mHealth interventions in improving medication adherence among people with hypertension: a systematic review. Curr Hypertens Rep. 2018;20(10):86. doi:10.1007/s11906-018-0886-7.
- McLean G, Band R, Saunderson K, Hanlon P, Murray E, Little P, McManus RJ, Yardley L, Mair FS. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600–12. doi:10.1097/HJH.0000000000000859.
- Rubinstein A, Miranda JJ, Beratarrechea A, Diez-Canseco F, Kanter R, Gutierrez L, Bernabé-Ortiz A, Irazola V, Fernandez A, Letona P, et al. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(1):52–63. doi:10.1016/S2213-8587(15)00381-2.
- Kario K, Nomura A, Harada N, Okura A, Nakagawa K, Tanigawa T, Hida E. Efficacy of a digital therapeutics system in the management of essential hypertension: the HERB-DH1 pivotal trial. Eur Heart J. 2021;42:4111–22. doi:10.1093/eurheartj/ehab559.