Abstract
145 fungal isolates were obtained from three sampling sites situated within the Nam River basin, located in the southern region of South Korea. Through ITS sequence analysis, the fungal isolates were identified to comprise 55 species of ascomycetes and 11 species of basidiomycetes. The 55 species of ascomycetes exclusively belong to the phylum Pezizomycotina, comprising 33 species of Dothideomycetes, 6 species of Eurotiomycetes, and 16 species of Sordariomycetes. Regarding their plant pathogenicity, an investigation into the fungi’s ability to penetrate solid media revealed Nigrospora chinensis as displaying the highest growth, followed by Pseudopestalotiopsis theae, various Curvularia species, Diaporthe species, and Alternaria alternata. Further research associating this penetration ability with fungal pathogenicity is deemed necessary. Among the 10 fungal species exhibiting penetration abilities, an examination of their capability to degrade biological polymers revealed that two strains of D. phaseolorum displayed exceptional polymer degradation. These strains exhibited remarkable abilities in decomposing malachite green and crystal violet, both recalcitrant dyes. This study underscores the potential utilization of fungal diversity in freshwater environments as a foundational approach to address freshwater pollution issues.
1. Introduction
Biodiversity provides various functions and productivity to the Earth’s environment and serves as a crucial element in the resilient adaptation of ecosystems to environmental changes [Citation1]. Fungi, primary decomposers in ecosystems, play a pivotal role as a key link in the global carbon cycle, returning carbon accumulated in plants back into the atmosphere. Paradoxically, their role intertwines with carbon emissions from fossil fuels, contributing to atmospheric carbon accumulation. Climate change due to atmospheric carbon accumulation significantly impacts fungal biodiversity. Fungi adapt their growth to habitat temperatures and are closely associated with plant ecosystems, making them susceptible to the effects of temperature rise and subsequent changes in plant ecology. The reduction in fungal diversity, the emergence of heat-adapted fungi, and the introduction of non-native fungal species from tropical and subtropical regions are expected to become major topics in the context of fungal diversity in the era of global warming. Consequently, comprehensive studies on fungal diversity and conservation across various habitats become essential to address these challenges.
In terrestrial ecosystems, freshwater habitats serve as reservoirs of fungal diversity, hosting over 3000 species, including various Ascomycetes, Basidiomycetes, Chytridiomycetes, and Oomycetes, in diverse freshwater environments [Citation2]. Although systematic studies on freshwater fungal habitats in Korea are limited, specific fungi such as Mortierella fluviae, Paraconiothyrium estuarinum, and Pyrenochaetopsis paucisetos have been identified in samples from the Yeongsan River and Suncheon Bay wetlands [Citation3,Citation4]. Additionally, widespread distribution of genera like Aspergillus, Cladosporium, Penicillium, Epicoccum, Paraconiothyrium, Septoriella, and Talaromyces, regardless of aquatic conditions or marine environment, have been reported [Citation5]. These fungal clusters exhibit diverse compositions and sizes based on seasonal and environmental variables, serving as valuable biological indicators of environmental changes [Citation6]. Furthermore, fungi isolated from ecosystems represent crucial resources due to their potential for various beneficial purposes. Species belonging to genera such as Trichoderma, Aspergillus, Fusarium, Penicillium, and Candida are utilized not only as indicators of environmental pollution but also to investigate the lifespan or persistence of pollutants, integrating past, present, and future ecological conditions [Citation7].
As mentioned above, fungi, as primary decomposers in ecosystems, externally produce various hydrolytic and oxidative enzymes. Among the hydrolytic enzymes produced by fungi are cellulase, protease, amylase, pectinase, and xylanase, which are utilized for breaking down biological polymers constituting plant matter. The oxidative enzymes primarily include laccase, lignin peroxidase, manganese peroxidase, and versatile peroxidase, which are extensively used in the breakdown of wood lignin and crystalline cellulose. The ability to decompose polymeric substances has long been a subject of applied research in various fields such as traditional food production, organic pollutant remediation in freshwater and soil environments, and the utilization of biomass. Fungi capable of degrading pollutants are diverse, including species like Phanerochaete chrysosporium, Lasiodiplodia sp., and Diaporthe schini [Citation8–11]. Recently discovered Diaporthe schini has been reported to efficiently adsorb and remove crystal violet (CV), a dye present in wastewater, at a high efficiency of 87% [Citation11]. Additionally, fungi such as A. fumigatus and A. niger have been reported to effectively decolorize CV [Citation12,Citation13]. Therefore, fungal diversity research holds significant importance not only in understanding fungi but also from the perspective of harnessing fungal resources.
The Nam River, originating from the Namdeogyu Mountains, is a tributary of the Nakdong River, extending 189.8 kilometers in length. Flowing through Jinju City, it serves as a national waterway and plays a critical role as a significant source of drinking water and industrial water in the western Gyeongsangnam-do region, South Korea. This study aimed to investigate fungi in the Nam River area, analyzing their physiological and biochemical characteristics to provide fundamental data regarding fungal diversity and applicability within the freshwater ecosystem of the Nam River region. Furthermore, this research explored the environmental remediation function of fungi in the Nam River basin by examining the degradation capabilities of isolated fungal strains using highly toxic and recalcitrant substances, malachite green (MG), and CV.
2. Materials and methods
2.1. Collection and isolation of fungi
Three locations around the Nam River (Location 1: Latitude 35.15311/Longitude 128.10396, Location 2: Latitude 35.181084/Longitude 128.097619, Location 3: Latitude 35.159235/Longitude 128.031318) were selected for the collection of airborne, aquatic, and marshland soil fungi from April to August 2022. Airborne fungi were collected by leaving DRBC agar plates (glucose 10 g/L, peptone 5 g/L, KH2PO4 1 g/L, MgSO4·6H2O 0.5 g/L, Rose Bengal 0.025 g/L, dichloran 0.002 g/L, chloramphenicol 0.1 g/L, and agar 1.5%) open for 5 min at each location. Aquatic fungi were collected by spreading samples obtained from the river on DRBC agar. Soil fungi were collected by suspending 0.1 g of soil sample in 1 ml of distilled water, centrifuging it, and then spreading the supernatant on DRBC agar. Fungi grown on DRBC were transferred to PDA and incubated at 25 °C for further cultivation.
2.2. ITS sequence analysis
The fungal mycelia grown on PDA medium were frozen in liquid nitrogen and ground into a fine powder using a mortar and pestle. Genomic DNA was then extracted using the Genomic DNA Prep Kit (BIOFACT, Daejeon, Korea). The extracted DNA was used as a template to amplify the internal transcribed spacer (ITS) region. The obtained sequences were subjected to BLAST analysis for fungal species identification. Samples obtained from different environments were labeled as A (air), W (water), or S (soil), and isolated fungal strains from the same location were numbered sequentially. The ITS sequences of the isolated fungi underwent multiple sequence alignment using the MAFFT service [Citation14], followed by the construction of a phylogenetic tree using the Neighbor-Joining method (with 1,000 Bootstrap resampling iterations). The generated phylogenetic tree was visualized using the Phylo.io program [Citation15], which was omitted here but summarized in .
2.3. Measurement of vertical growth
To assess the ability of isolated strains to colonize substrates, their vertical growth on a solid medium was measured. PDA was poured into 15-ml culture tubes, and the fungal mycelium of selected strains was inoculated. After culturing for a total of 21 d, the length of mycelial growth was measured from each sample. For further experimentation with selected strains, PDA was loaded into serological pipette tips (25 ml, 34.5 cm), and the mycelium was inoculated vertically. The mycelial growth was then measured over a period of 28 d.
2.4. Degradation of biological polymers
The ability to degrade biological polymers such as cellulose, starch, skim milk, polygalacturonic acid, and xylan, was assessed by measuring fungal growth on minimal medium (Congo Red 1 g/L, yeast nitrogen base 1 g/L, and agar 1.5%) supplemented with each substrate at a concentration of 5 g/L [Citation16,Citation17]. The degradation capacity of malachite green (MG) and crystal violet (CV) was observed by adding concentrations ranging from 10 to 40 ppm of each dye to PDA and culturing at 25 °C.
3. Results
3.1. Partial identification by ITS sequence analysis
The total number of isolated fungal strains at three locations in the Nam River basin amounted to 145 strains (). Among these, airborne fungi totaled 82 strains, soil-based fungi comprised 27 strains, and water-based fungi accounted for 36 strains. After excluding duplicates in the analysis of their ITS sequences for partial identification, a final count of distinct species amounted to 66 (). Of these, 11 were Basidiomycetes, while 55 belonged to Ascomycetes, indicating significantly higher diversity within the Ascomycetes group. The isolated Basidiomycete strains were observed to belong to the Agaricomycetidae, Polyporales, and Russulales, typical mushroom strains found in South Korea’s general environment. For instance, Emmia lacerate (W1_3 and W1_6) was identified in respiratory infections among domestic hospital patients [Citation18], while Phanerochaete concrescens was reported as an endophytic fungus in pine trees from domestic mountainous regions [Citation19]. Peniophora incarnata (W1_9) has been noted for its Mn-peroxidase, which effectively degrades recalcitrant substances like anthracene [Citation20].
Table 1. List of fungal isolates.
The 55 species of isolated Ascomycetes all belong to the phylum Pezizomycotina, comprising 33 species of Dothideomycetes, 6 species of Eurotiomycetes, and 16 species of Sordariomycetes upon analysis. Based on the phylogenetic tree analysis using ITS sequences, among the Dothideomycetes, fungi belonging to the Dothideomycetidae subclass, such as Cladosporium cladosporioides (A1_6, five isolated strains), Cladosporium halotolerans (W1_7), Cladosporium tenuissimum (W3-3 and W3_7), often reported as plant pathogenic fungi [Citation21–23], were grouped with fungi from the Eurotiomycetes and Sordariomycetes, indicating that the ITS sequence-based phylogenetic tree did not accurately reflect the evolutionary relationships among these fungi (). This phenomenon has been observed in other fungal isolation studies as well [Citation24]. Microdiplodia miyakei (A3_9) has been observed in dust samples from North America but has not been reported in South Korea [Citation25]. Among the Dothideomycetes, the fungi belonging to the Pleosporomycetidae subclass were the most abundant, with 29 species. This group includes fungi reported in literature such as Alternaria alternata (A1_1, A1_2, and A1_3), Periconia macrospinosa (A3_20), Curvularia intermedia (A2_2), Corynespora cassiicola (A3_1) as plant pathogens [Citation23,Citation26,Citation27], and Phoma herbarum (A3_4) known for synthesizing Gibberellin [Citation28].
3.2. Vertical growth characteristics
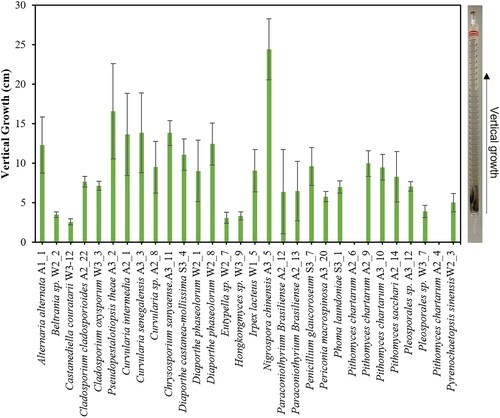
Upon observing the growth of fungi in petri dishes containing PDA medium, it was noted that a total of 30 fungal strains deeply penetrated the surface of the solid medium, growing toward the bottom. Most of these strains, considered predominantly plant-pathogenic fungi, exhibited the ability to penetrate the inner layers of the solid medium, akin to their capacity to invade plant tissues. To assess this similarity, PDA was filled into a serological pipette (25 ml), and after 28 days of cultivation, the length of growth was measured (). Nigrospora chinensis (A3_5) displayed the most substantial vertical growth, reaching 24.4 cm from the inoculation point, followed by Pseudopestalotiopsis theae (A3_2), Curvularia species (A2_1, A3_3, and A2_8), Diaporthe species (S3_4, W2_1, and W2_8), and Alternaria alternata (A1_1), showing the next highest growth rates. In South Korea, N. chinensis has been isolated as an endophyte or plant pathogen on woody plants [Citation29], while various Nigrospora species have recently been discovered in coastal algae [Citation30]. This fungus is reported to produce various substances from the terpenoid group [Citation31,Citation32]. Meanwhile, Beltrania sp. (W_2-2) has been previously isolated as an endophyte in fir needles in South Korea [Citation33], but in a solid medium, it predominantly grew near the surface. Among the Pithomyces chartarum strains, strains A2_4 and A2_6 did not penetrate into the medium, whereas strains A2_9 and A3_10 exhibited relatively better penetration. The ability of fungal mycelia to penetrate the interior of solid organic matter is an essential characteristic for both endophytic and saprophytic fungi. Mycelia penetrating the substrate absorb nutrients at their tips, transport intracellular substances from rear hyphae to tips, and facilitate gas exchange for respiration, necessitating efficient oxygen-carbon dioxide exchange at depth. Therefore, a more in-depth investigation is required to understand the solid medium penetration ability of these fungi from the perspective of material movement and exchange.
Figure 1. Vertical growth characteristics of the fungal isolates. Fungal isolates were inoculated at the tip of the serological pipette (25 ml, 34.5 cm) which was filled with PDA medium. The vertical growth was measured from the tip to the mycelial front propagated inside the pipette after 3 weeks of incubation at 25 °C.
3.3. Degradation of biological polymers and recalcitrant
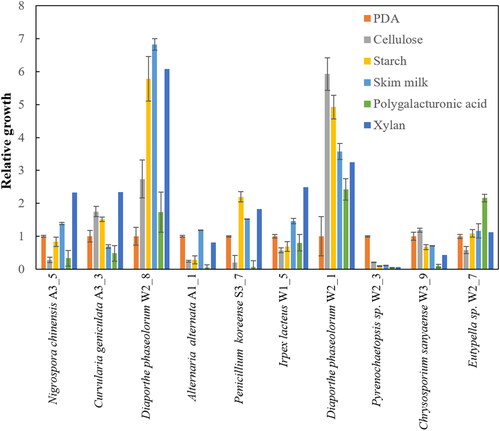
The ability to penetrate solid media is presumed to be closely related to fungi’s capacity for material degradation. To investigate this, the ability of 10 fungal strains to degrade various biological polymers such as cellulose, starch, skim milk, polygalacturonic acid, and xylan was measured by comparing the growth on minimal media to their growth on PDA. The results indicated that the growth of most strains was similar on both PDA and minimal media. However, in the case of Diaporthe phaseolorum W2_8 and W2_1 strains, their growth on biological polymers was over 3-7 times superior compared to their growth on PDA (). This suggests that the ability to penetrate solid media and the capacity for material degradation might not have a direct correlation, indicating that within the fungal mycelium, the movement of substances like oxygen through cellular connections might be more critical.
Figure 2. Growth of the fungal isolates on the minimal agar plates containing 5 g/L biological polymers, including cellulose, starch, skim milk, poly galacturonic acid and xylan. The relative growth was calculated by dividing the mycelial colony diameter grown on the biological polymers by the mycelial diameter grown on PDA.
Meanwhile, since the strains W2_8 and W2_1 demonstrated exceptionally superior abilities in degrading biological polymers, their potential for decomposing recalcitrant substances, such as MG and CV, was investigated by adding 40 ppm of MG and CV to composite media. The results revealed that most fungi could not grow due to the toxicity of MG and CV. However, the strains W2_8 and W2_1 were able to decompose and grow in the presence of MG and CV similar to their capabilities in degrading biological polymers ()
Figure 3. Degradation of malachite green (MG) and crystal violet (CV) by the fungal isolates. (A) Growth of some selected fungal isolates on PDA containing 40 ppm MG or CV. The plates were incubated 2 weeks at 25 °C. (B) Growth of Diaporthe phaseolorum W2_8 or W2_1 in different concentration of MG or CV. (C) Degradation of MG and CV by D. phaseolorum W2_8 or W2_1 in liquid culture. The mycelial cell were grown in PDA medium containing 20 ppm of MG or CV for 21 d at 25 °C. The culture supernatants, clarified by centrifugation for 5 min at 3000 rpm, were subjected to the UV-Vis spectral analysis.
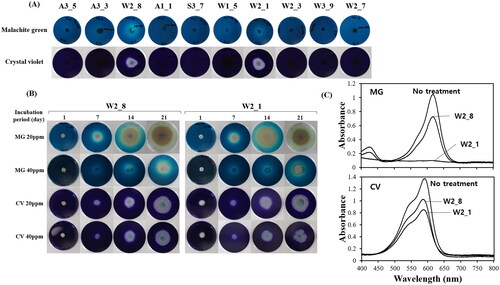
Based on these findings, the decomposition extent of MG and CV by the strains W2_8 and W2_1 was further investigated at concentrations of 20 ppm and 40 ppm over time. Both strains almost completely decomposed 20 ppm MG after 21 d of cultivation. At a concentration of 40 ppm MG, their growth was slower due to higher toxicity (). The degradation of MG by fungi is presumed to be mediated by oxidative enzymes secreted by fungi, as the surrounding area becomes transparent as MG breaks down. Enzymes such as laccase and peroxidase, known as oxidative enzymes produced by fungi, are thought to be responsible for MG decomposition [Citation34]. CV, similar to MG, is a triphenylmethane dye. Unlike MG, fungi internalize and decompose CV within their mycelial cells without displaying a clear halo around the colonies.
Subsequently, 20 ppm concentrations of MG and CV in liquid media were examined for their decomposition using spectrophotometric analysis (). Analyzing the absorption spectra of the cultivation medium after 20 days revealed that the strain W2_1 completely decomposed MG, while the strain W2_8 decomposed only about 30%. For CV, approximately 61% and 77% remained in the strains W2_1 and W2_8, respectively, indicating that W2_1 had a slightly higher decomposition ability compared to W2_8, though lower than that observed for MG.
4. Discussion
The diverse fungi in freshwater ecosystems play a crucial role in organic matter decomposition and environmental purification. Therefore, the diversity of fungi in freshwater ecosystems can serve as an indicator of the health of these aquatic systems. However, studies on fungal diversity can vary significantly depending on climate conditions, physicochemical characteristics of sampling locations, sampling methods, sample processing, and culture methods. Unlike biodiversity studies of visible fauna and flora, studying fungal diversity is quite challenging. In an effort to investigate fungal diversity in freshwater systems, this study aimed to isolate fungi from air, soil, and water samples in the Nam River basin using a DRBC medium and analyzed their diversity through ITS sequencing. As a result, a total of 145 strains were isolated from three locations, revealing the presence of 66 species consisting of 55 ascomycetes and 11 basidiomycetes. The number of these fungal species is likely underestimated as it is limited to culturable fungi on DRBC, indicating a potentially larger fungal diversity overall.
During the cultivation of isolated fungi, approximately 30 strains were discovered capable of penetrating and deeply infiltrating the interior of solid media, most of which were plant pathogenic fungi. Assessing their ability to penetrate solid media revealed that fungi known for their plant pathogenicity [Citation29] such as N. chinensis, P. theae, Curvularia, and A. alternata exhibited high penetrating growth capabilities. This suggests a potential correlation between fungal plant pathogenicity and their ability for invasive mycelial growth, although further rigorous experimentation is required to substantiate this.
The material degradation capacity of fungi is associated with their production of extracellular hydrolytic and oxidative enzymes, which are linked to the organic matter degradation in freshwater ecosystems. Investigating the degradative abilities of biological polymers such as cellulose, starch, skim milk, polygalacturonic acid, xylan, and the toxic triphenylmethane dyes, MG and CV, revealed that D. phaseolorum strains W2_8 and W2_1, revealed to have high biological polymer degradation activity, effectively decomposed MG and CV. D. phaseolorum is a fungus causing stem canker in soybeans and plant stems, with its metabolites known to induce seedling inhibition and wilting diseases in plants [Citation35–37]. Its related species, D. longicolla, has been reported to secrete laccase enzymes capable of decomposing the persistent environmental hormone bisphenol A [Citation38,Citation39], while D. schini efficiently adsorbs and removes CV found in dye-contaminated wastewater [Citation11]. Recent discoveries have revealed the production of lignin-degrading enzymes such as lignin peroxidase, Mn peroxidase, and laccase by these fungi [Citation34]. Consequently, the degradation of MG and CV by D. phaseolorum observed in this study is likely attributed to the lignin-degrading enzymes produced by these fungi. In conclusion, this research marks the initial paper shedding light on the fungal diversity in the Nam River basin, potentially contributing to the study of fungal diversity in domestic freshwater environments and aiding research on environmental purification using freshwater fungi.
Author contributions
YJC and HSR conceived the project. JYP, JLP, and SC conducted sampling, isolation, characterization, and PCR analysis. YJC, HE, and HSR prepared the manuscript. All the authors have read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zedda L, Rambold G. The diversity of lichenised fungi: ecosystem functions and ecosystem services. In: Upreti D, Divakar P, Shukla V, Bajpai R, editors. Recent advances in lichenology: modern methods and approaches in lichen systematics and culture techniques. New Delhi: Springer; 2015. pp. 121–145. doi: 10.1007/978-81-322-2235-4_7.
- Tsui CKM, Baschien C, Goh TK. Biology and ecology of freshwater fungi. In: Li DW. editor. Biology of microfungi: Fungal biology. Cham: Springer; 2016. doi: 10.1007/978-3-319-29137-6_13.
- Nguyen T, Thuong T, Lee HB. Characterization of a zygomycete fungus, Mortierella minutissima from freshwater of Yeongsan river in Korea. Kor J Mycol. 2016;44(4):346–349.
- Goh J, Jeon YJ, Mun HY, et al. Isolation and characterization of eleven unrecorded Pezizomycotina species from freshwater ecosystems in Korea. Kor J Mycol. 2020;48(4):423–443.
- Park JM, Hong JW, You YH, et al. Endophytic fungi of emersed halophytes in river deltas and tidal flats of the Korean Ramsar wetlands. JMSE. 2021;9(4):430. doi: 10.3390/jmse9040430.
- Choi TJ, Malik A, An HE, et al. Seasonal diversity of microeukaryotes in the Han river, Korea through 18S rRNA gene metabarcoding. Evol Bioinform. 2022;18:1–10.
- Zaghloul A, Saber M, Gadow S, et al. Biological indicators for pollution detection in terrestrial and aquatic ecosystems. Bull Natl Res Cent. 2020;44(1):127. doi: 10.1186/s42269-020-00385-x.
- Aust SD. Degradation of environmental pollutants by Phanerochaete chrysosporium. Microb Ecol. 1990;20(1):197–209. doi: 10.1007/BF02543877.
- Reddy CA. The potential for white-rot fungi in the treatment of pollutants. Curr Opin Biotechnol. 1995;6(3):320–328. doi: 10.1016/0958-1669(95)80054-9.
- Arunprasath T, Sudalai S, Meenatchi R, et al. Biodegradation of triphenylmethane dye malachite green by a newly isolated fungus strain. Biocatal Agr Biotechnol. 2019;17:672–679. doi: 10.1016/j.bcab.2019.01.030.
- Grassi P, Reis C, Drumm FC, et al. Biosorption of crystal violet dye using inactive biomass of the fungus Diaporthe schini. Water Sci Technol. 2019;79(4):709–717. doi: 10.2166/wst.2019.091.
- Al-Jawhari IFH. Decolorization of methylene blue and crystal violet by some filamentous fungi. Int J Environ Bioremed Biodegrad. 2015;3(2):62–65.
- Ali HM, Shehata SF, Ramadan KMA. Microbial decolorization and degradation of crystal violet dye by Aspergillus niger. Int J Environ Sci Technol. 2016;13(12):2917–2926. doi: 10.1007/s13762-016-1117-x.
- Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20(4):1160–1166. doi: 10.1093/bib/bbx108.
- Robinson O, Dylus D, Dessimoz C. Phylo.io: interactive viewing and comparison of large phylogenetic trees on the web. Mol Biol Evol. 2016;33(8):2163–2166. doi: 10.1093/molbev/msw080.
- Kim JY, Kwon HW, Tang L, et al. Analysis of the effect of media types and chromagenic chemicals on the detection of extracellular laccase activity among Lentinula edodes strains. Kor J Mycol. 2011;39(1):48–52. doi: 10.4489/KJM.2011.39.1.048.
- Kwon HW, Kim JY, Ko HG, et al. Assessment of the ability of extracellular enzyme production in hybrid strains of Lentinula edodes by chromogenic reaction-based plate assay. Kor J Mycol. 2011;39(2):99–104. doi: 10.4489/KJM.2010.39.2.099.
- Lee JM, Han E, Kim J, et al. Five korean cases of respiratory tract infection by filamentous basidiomycetes. Ann Lab Med. 2020;40(1):84–87. doi: 10.3343/alm.2020.40.1.84.
- Eo JK, Park H, Eom AH. Diversity of endophytic fungi isolated from Pinus densiflora and Juniperus rigida distributed in Mt. Baekryeonsan and Mt. Johangsan, Korea. Kor J Mycol. 2018;46(4):437–446.
- Lee AH, Kang CM, Lee YM, et al. Heterologous expression of a new manganese-dependent peroxidase gene from Peniophora incarnata KUC8836 and its ability to remove anthracene in Saccharomyces cerevisiae. J Biosci Bioeng. 2016;122(6):716–721. doi: 10.1016/j.jbiosc.2016.06.006.
- Nam MH, Park MS, Kim HS, et al. Cladosporium cladosporioides and C. tenuissimum cause blossom blight in strawberry in Korea. Mycobiology. 2015;43(3):354–359. doi: 10.5941/MYCO.2015.43.3.354.
- Lee W, Kim JS, Seo CW, et al. Diversity of Cladosporium (Cladosporiales, Cladosporiaceae) species in marine environments and report on five new species. MycoKeys. 2023;98:87–111. doi: 10.3897/mycokeys.98.101918.
- Ahn GR, Kim JE, Oh YS, et al. Undescribed fungal species of Eupenicillium, Mortierella, and Trichoderma isolated in the vicinity of demilitarized zone in Yeoncheon-gun, Gyeonggi-do. Korea. Kor J Mycol. 2018;46(4):359–367.
- Duan Y, Wu F, He D, et al. Diversity and spatial–temporal distribution of airborne fungi at the world culture heritage site Maijishan grottoes in China. Aerobiologia. 2021;37(4):681–694. doi: 10.1007/s10453-021-09713-8.
- Dietzel K, Valle D, Fierer N, et al. Geographical distribution of fungal plant pathogens in dust across the United States. Front Ecol Evol. 2019;7:304. doi: 10.3389/fevo.2019.00304.
- Lee JA, Lee SY, Choi YJ. Leaf blight caused by Curvularia intermedia on the invasive weed Lactuca serriola in korea. Kor J Mycol. 2023;51:246.
- Kwon MK, Yang KY, Cho BH. A target leaf spot disease caused by Corynespora cassiicola on cucumber cultivated in green house. Res Plant Dis. 2004;10(2):121–125. doi: 10.5423/RPD.2004.10.2.121.
- Hamayun M, Khan SA, Khan AL, et al. Phoma herbarum as a new gibberellin-producing and plant growth-promoting fungus. J Microbiol Biotechnol. 2009;19(10):1244–1249.
- Lee DJ, Lee JS, Lee HB, et al. Four endophytic ascomycetes new to korea: Cladosporium anthropophilum, C. pseudocladosporioides, Daldinia eschscholtzii, and Nigrospora chinensis. Kor J Mycol. 2019;47:187–197.
- Lee W, Kim DG, Perera RH, et al. Diversity of Nigrospora (Xylariales, Apiosporaceae) species identified in Korean macroalgae including five unrecorded species. Mycobiology. 2023;51(6):401–409. doi: 10.1080/12298093.2023.2283272.
- Wang H, Sang Z, Chen Y, et al. The chemical constituents of endophytic fungus Nigrospora chinensis of Gannan navel orange. Nat Prod Res. 2022;38(3):530–538. doi: 10.1080/14786419.2022.2125969.
- Wang B, Li H, Chen T, et al. Two new sesquiterpene derivatives, dendocarbin B and bisaborosaol C with antifungal activity from the endophytic fungus Nigrospora chinensis GGY-3. Nat Prod Res. 2022:1–9. doi: 10.1080/14786419.2022.2151011.
- Kim CK, Eo JK, Eom AH. Molecular identification of endophytic fungi isolated from needle leaves of Pinus thungergii. Kor J Mycol. 2012;40(4):183–186. doi: 10.4489/KJM.2012.40.4.183.
- Kumar V, Prasher IB. Ligninolytic enzymes production by endophytic fungus Diaporthe phaseolorum (Desm.) Sacc. under the influence of different carbon and nitrogen sources. SIF. 2021;6(1):531–542. doi: 10.5943/sif/6/1/43.
- Pioli R, Gattuso S, Prado D, et al. Recent outbreak of stem canker (Diaporthe phaseolorum var. meridionalis) of soybean in Santa Fe, Argentina. Plant Dis. 1997;81(10):1215–1215. doi: 10.1094/PDIS.1997.81.10.1215A.
- Ivanovic M, Sinclair JB. Comparison of possible phytotoxic metabolites in culture filtrates of the Diaporthe/Phomopsis complex of soybeans. Mycopathologia. 1989;108(1):59–63. doi: 10.1007/BF00436785.
- Parveen S, Wani AH, Bhat MY. Effect of culture filtrates of pathogenic and antagonistic fungi on seed germination of some economically important vegetables. Braz J Biol Sci. 2019;6(12):133–139. doi: 10.21472/bjbs.061212.
- Baluyot JC, Santos HK, Batoctoy DCR, et al. Diaporthe/Phomopsis longicolla degrades an array of bisphenol analogues with secreted laccase. Microbiol Res. 2022;257:126973. doi: 10.1016/j.micres.2022.126973.
- Mani S, Bharagava RN. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev Environ Contam Toxicol. 2016;237:71–104. doi: 10.1007/978-3-319-23573-8_4.

