 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In recent decades, an enormous potential of fungal-based products with characteristics equal to, or even outperforming, classic petroleum-derived products has been acknowledged. The production of these new materials uses mycelium, a root-like structure of fungi consisting of a mass of branching, thread-like hyphae. Optimizing the production of mycelium-based materials and fungal growth under technical conditions needs to be further investigated. The main objective of this study was to select fast-growing fungi and identify optimized incubation conditions to obtain a dense mycelium mat in a short time. Further, the influence of the initial substrate characteristics on hyphae expansion was determined. Fungal isolates of Ganoderma lucidum, Pleurotus ostreatus, and Trametes versicolor were cultivated for seven days on substrate mixtures consisting of various proportions of pine bark and cotton fibers. Furthermore, the substrates were mixed with 0, 2, and 5 wt.% calcium carbonate (CaCO3), and the incubator was flushed with 0, 5, and 10 vol.% carbon dioxide (CO2). All samples grew in the dark at 26 °C and a relative humidity of 80%. Evaluation of growth rate shows that cotton fiber-rich substrates performed best for all investigated fungi. Although Pleurotus ostreatus and Trametes versicolor showed comparatively high growth rates of up to 5.4 and 5.3 mm d−1, respectively, mycelium density was thin and transparent. Ganoderma lucidum showed a significantly denser mycelium at a maximum growth rate of 3.3 mm d−1 on a cotton fiber-rich substrate (75 wt.%) without CaCO3 but flushed with 5 vol.% CO2 during incubation.
1. Introduction
According to a recent estimate, from 1950 to 2015, about 9150 tons of primary plastics were produced, accumulating about 6945 million tons of plastic waste on the earth’s surface. Of the total waste generated, only 9% was recycled, 12% incinerated, and 79% was amassed in landfills and other terrestrial and marine environments [Citation1]. Single-use products, such as polystyrene and polypropylene, are widely used in various industries, especially for packaging materials, due to their light weight and strength of shape [Citation2]. However, owing to its chemical properties, polystyrene is not biodegradable, and due to its energy-intensive and expensive recycling procedure, its use results in the production of solid waste disposed of in landfills.
Newly developed bio-based materials can reduce environmental impacts and provide a solution for addressing conventional packaging sustainability challenges [Citation3]. One of these promising materials is mycelium composites. Such two compound materials consist of the vegetative part of fungal organisms, the so-called mycelium. It penetrates and partially consumes its surrounding substrate and acts as a natural binder, subsequently enveloping and crosslinking the substrate and additive particles [Citation4]. When reaching complete substrate colonization, growth is inhibited above a critical temperature to render the material inert and allow the evaporation of the residual water. The final material resembles properties similar to petrochemical foams or particle boards, depending on the post-processing, such as the drying procedure and hot and cold-pressing [Citation5]. As a result, the materials can be used in a wide range of applications, such as packaging [Citation6] or thermal and acoustic insulation [Citation7,Citation8].
The advantages offered by these composites include their positive thermal and acoustic properties, fire safety and ability to upcycle mineral-, forestry-, and agricultural waste [Citation9,Citation10]. Furthermore, the composites are biodegradable under industrial composting conditions within 12 weeks due to their exclusively biogenic raw materials [Citation11].
There are three main groups of wood-decaying fungi: soft, brown, and white rot fungi [Citation12]. The latter is characterized by the singular ability to complete lignin degradation and excellent decay of wood polysaccharides [Citation13]. Using phenol-oxidizing and peroxidase-producing enzymes, fungi depolymerize and mineralize complex macromolecules [Citation14]. Therefore, the chemical structure of lignin changes during the enzymatic oxidation with laccase and peroxidase [Citation15,Citation16]. During this process, lignin-based radicals can be crosslinked, forming an adhesive between the fibers [Citation14]. Among others, the mycelium species is one of the main factors affecting the properties of mycelium composites and, therefore, their field of application [Citation17,Citation18].
Currently, 36 fungal species have been used or are mentioned in patents for mycelium composites [Citation19]. Ganoderma lucidum, Pleurotus ostreatus, and Trametes versicolor are three of mycelium composite production’s most recently used white-rot fungi. Their growth conditions, such as preferred nutrient medium and temperature, have already been investigated. According to Jo et al. [Citation20], G. lucidum grows the fastest as incubated at 25–30 °C and on potato dextrose agar media with a chemically increased pH up to 9 by addition of sodium hydroxide. Requirements in terms of temperature and media are similar for T. versicolor [Citation21]. Additives, such as 2 wt.% calcium carbonate (CaCO3), are already used in mycelium cultivation to elevate the initial pH chemically [Citation22] since the hyphal branching increases as calcium sources are added at inoculation [Citation23]. However, the correlation between pH and growth behavior has not yet been fully explored.
Furthermore, a positive effect of up to 29 vol.% carbon dioxide (CO2) in the environment during incubation was found for P. ostreatus [Citation24]. Elevating the CO2 concentration to 17 vol.% leads to an increased radial mycelium growth by 25%. However, the effect of CO2 on other fungi, such as G. lucidum and T. versicolor, is scarcely investigated or described in the context of mycelium composite production. Further, used raw materials for mycelium composite production are not examined thoroughly, although substrate selection influences both the growth behavior of the mycelium and, subsequently, the material’s properties [Citation25,Citation26]. A general understanding of chemical and physical substrate characteristics and related growth behavior of mycelium needs to be improved.
Three fungi were used in this study to investigate their growth behavior and, thus, their suitability for mycelium composite production. Five mixing ratios of lignin and cellulose-rich substrates were used to better understand the required composition of biomass for sufficient mycelium formation. Further, pH was chemically elevated using CaCO3 in three concentrations (0, 2, and 5 wt.%). The effect of CO2 on mycelial growth was tested by flushing the incubator (0, 5, and 10 vol.%).
2. Materials and methods
2.1. Overview of conducted experiments
This research investigates the effects of different lignocellulosic substrate mixtures on the growth behavior of three fungal species, namely G. lucidum, P. ostreatus, and T. versicolor. Comprehensive proximate and elemental analyses were conducted to understand the requirements of micro- and macronutrients better. Further, the impact of a chemically elevated pH by adding 2 and 5 wt.% CaCO3 was tested. The incubator was flushed with 5 and 10 vol.% CO2 to test its influence on the radial expansion rate (mm d−t) and mycelium density. In total, 135 combinations of substrate, fungi, buffer addition, and CO2 flush were conducted in triplicate and analyzed. The experiments determined culture conditions for improved mycelium cultivation.
2.2. Fungal species
Ganoderma lucidum, P. ostreatus, and T. versicolor were obtained from the Institute of Food Chemistry of the Leibnitz University Hannover in Germany. An overview, including abbreviations used in this study, is listed in .
Table 1. List of fungal species used in this study.
2.3. Substrates
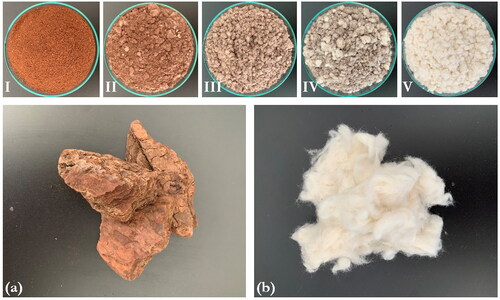
Two different types of substrates were used to determine the optimal characteristics for the growth of the fungi and their effect on the degradation of lignin and cellulose. Cotton fibers were selected as a model substance for material rich in cellulose (provided by Pat & Patty, Hürtgenwald, Germany). Pine bark was selected as a model substance for lignin-rich material (provided by Plantura GmbH, Munich, Germany). Before the experiments, both substrates were dried in an oven at 105 °C for 24 h, ground with a cutting mill and sieved to obtain a homogenous particle size of 0.5–1 mm. Then, the substrates were mixed in various compositions from 100% pine bark to 100% cotton fiber ().
Figure 1. Top row: milled substrate mixtures of pine bark and cotton fibers in different weight proportions; lower row: (a) raw pine bark; (b) cotton fibers used for this study.
The moisture content (MC) and ash content (AC) were determined in triplicate for initial biomass according to DIN EN 14774-3:2010-02 and DIN EN 14775:2010-04, respectively. Then, dry matter (DM) content and organic dry matter (oDM) content were calculated.
The total carbon (TC) content and total Kjeldahl nitrogen (TN) were measured with multi N/C 3100 (Analytik Jena GmbH& Co. KG, Jena, Germany) and Vapodest 50S (C. Gerhardt GmbH & Co. KG, Königswinter, Germany), respectively. Forth following, the carbon to nitrogen (C/N) ratio was calculated. The elemental composition of the samples was analyzed using an ICP-MS (Nexion2000, Perkin Elmer Inc., Waltham, MA).
The pH measurement of solids was carried out by adding 200 ml Millipore-Q-water (<0.05 µS) to 2 g substrate and continuously shaking for 60 min (IKA KS 260 control, IKA®-Werke GmbH & Co. KG, Staufen, Germany). Afterwards, the pH was measured using a pH meter (GE 114 BNC, Senseca, Regenstauf, Germany). The same procedure was conducted by mixing substrates I–V with 2 and 5 wt.% CaCO3, respectively, to determine the effect of an elevated pH.
The mixtures’ water capacity (U) was determined by adapting the procedure of Alemu et al. [Citation27] by adding 50 ml Millipore-Q-water to 5 g of each substrate and covered with aluminum foil to avoid moisture loss by evaporation. The samples were soaked for 24 h at ambient conditions. Afterwards, residual water could drip off through sieves for 2 h. Then, the crucibles containing the wet biomass were weighed again. Finally, the water capacity was calculated as follows:
(1)
(1)
where Ww is the wet weight (g) and Wd is the dry weight (g).
2.3.1. Mycelium cultivation
Prior to the experiments, MEA agar plates (mixture consisting of 15 g agar, 30 g malt extract, 5 g soy peptone, and 1000 ml Millipore-Q-water) were inoculated with a pre-grown mycelium disk of 10 mm in diameter, placed in the center of the plates. The Petri dishes were sealed with parafilm to avoid cross-contamination in the incubator (ICH110, Memmert GmbH & Co. K, Schwabach, Germany) and stored at 26 °C and 80% relative humidity with no additional flush of CO2. After growth, they were kept in the fridge at 4 °C until use, but maximum for 30 days.
2.3.2. Test implementation
For each experiment, 5 g of the dry substrate mixture was weighed in a glass Petri dish. Those samples investigating the effect of an elevated initial pH were mixed with 2 or 5 wt.% pre-dried CaCO3 powder, respectively. An overview of the substrate mixture composition and CaCO3 addition is shown in . Then, the previously determined mass of Millipore-Q-water was slowly poured over the substrate. The mixture was allowed to soak in for approximately 1 h under ambient conditions. All prepared samples were autoclaved at 121 °C for 50 min (CertoClav VacuumPro 8-22L, CertoClav Sterilizer GmbH, Leonding, Austria) to eliminate any competing microorganisms that might hinder mycelial development.
Table 2. List of substrate mixtures and additives used in this study.
The Petri dishes were immediately closed to avoid water loss by evaporation. They were allowed to cool down in front of the sterile laminar flow hood for approximately 30 min. Before inoculation, a metal stamp was used to compress the substrates and obtain an even surface. Then, the prepared mycelium dishes were used to inoculate the prepared substrate mixtures with a mycelium disk, cut out using a cork borer, 10 mm in diameter and placed in the center of the plates. Lastly, the samples were stored for seven days in the incubator at 26 °C and 80% relative humidity with 0, 5, and 10 vol.% CO2 flush, respectively.
Radial expansion was measured every 24 h by measuring the distance from the inoculum disk to the most distant visible hyphae on the photo in triplicate.
After seven days of incubation, the average radial expansion (mm d−m) was calculated, and the colony radius was determined. Lastly, the Petri dishes were removed from the incubator. Mycelial density was evaluated visually and rated following a scale ranging from T (thin, almost transparent), ST (somewhat thin), SC (somewhat compact) to C (compact and firm, white), as previously described by Jo et al. [Citation20]. Dried-out samples are marked with D.
3. Results and discussion
3.1. Feedstock characteristics and chemical composition
Overall, the five substrate mixtures differed in the chemical composition (). Substrates with a high share of pine bark have higher TC and TN content than those rich in cotton fibers. Accordingly, the highest TC and TN content is found for substrate I with 56 and 0.46 wt.%, respectively, which are 12 and 56% higher than in substrate V. Consequently, the C/N ratio of 121 is significantly lower in substrate I and increases with the cellulose content in the substrate mixture. Substrate V, therefore, has a 73% higher C/N ratio of 166. The pH value is also dependent on the substrate. Mixtures I and II, exclusively and primarily consisting of pine bark, have values in the acidic range of 4.73 and 5.00, respectively. In contrast, with a high proportion of cellulose, the value rises up to 8.43 and is, therefore, in the alkaline range.
Table 3. Determined chemical characteristics and calculated C/N ratio of the investigated substrates.
All mixtures have a very high oDM content, so the AC is correspondingly low. Pure cotton fibers have the lowest, containing <1 wt.% ash. The more pine bark is added, the higher the AC; pure bark substrate is one and a half times as high at 1.62 wt.% but generally relatively low for biomass. The initial U varies from 80 to 90%, which is comparatively high. Its value increases with higher cellulose fiber content.
As shown in , all substrate mixtures provide a broad spectrum of macro- and micronutrients required for mycelium culture, but their proportions differ considerably from one mixture to another. The K content increases with higher proportions of cotton fibers to a value of up to 3166 mg kg−g DM, two and a half times the measured K in pine bark. The opposite was observed for Ca, Mg, and Na, as the addition of cotton fibers decreased their proportions. Consequently, the highest measured values for Ca, Mg, and Na are found in the pine bark substrate at 2669, 491, and 155 mg kg−g DM, respectively. Even though only scarce amounts of Mn were measured in substrates I–V at 74–5 mg kg−g DM, the difference is enormous. Accordingly, the content of this micronutrient can be increased by up to 14 times if the proportion of pine bark in the substrate mixture is increased. The content of P and Fe varies from substrate to substrate, so it is impossible to make a uniform statement about the influence of the proportion of pine bark or cotton fibers on the nutrients. The initial substrates have P contents between 254 and 317 mg kg−g DM for mixtures I and II, respectively. The Fe content was in a similar range, around 210 mg kg−g DM for all mixtures, although there is also a relatively high standard deviation here.
Figure 2. Elemental composition of the substrates I–V in terms of potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P), iron (Fe), sodium (Na), manganese (Mn), zinc (Zn), copper (Cu), nickel (Ni), chromium (Cr), cobalt (Co), and lead (Pb) (mg kg−1 DM); n = 3.
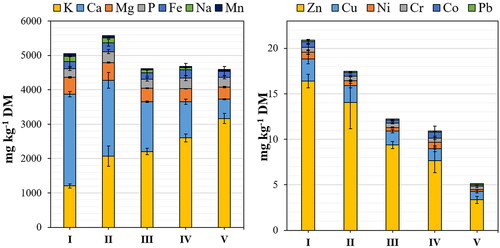
The more cotton fibers are added to the substrate mixture, the lower the Zn content. As a result, it is about five times higher in substrate I with 16 mg kg−g DM than in substrate V with only 3 mg kg−g DM. The situation is similar to the content of the micronutrient Cu, which is about twice as high in pure pine bark substrate than in pure cotton fiber. Ni, Cr, Co, and Pb are present in all substrate mixtures in very low quantities of >1 mg kg−g.
3.1.1. Radial expansion of mycelium
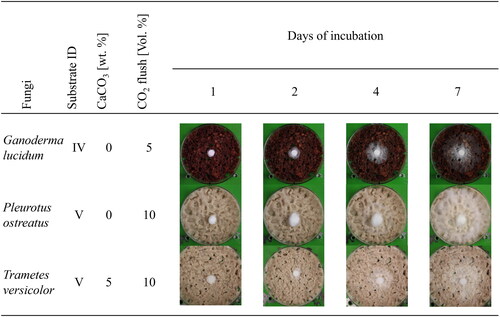
During the growth period in the incubator, photos were taken daily to evaluate the radial expansion of the mycelium. shows sample images of the three fungi examined after 1, 2, 4, and 7 days. It can be clearly seen that all fungi already formed fine, visible mycelium from the edge of the inoculation disk on the first day in the incubator. Interestingly, P. ostreatus forms not only mycelium, which begins to colonize the substrate below but also hyphae, which grow vertically toward the Petri dish lid. On the fourth day of incubation, all fungi continued growing, covering the substrate with somewhat thin (G. lucidum) and thin (P. ostreatus and T. versicolor) mycelium. In the case of G. lucidum, on day 7 of incubation, it is noticeable that although the mycelium has continued to grow radially, the mycelium density is significantly lower than on the previous days. As a result, the younger mycelium is only very finely visible on the surface of the substrate. Although P. ostreatus has evenly covered the entire substrate surface with mycelium after seven days of growth in the incubator, the mycelium density is still low. Trametes versicolor behaves similarly, but after seven days of growth, only about half of the substrate surface is covered with very thin mycelium.
Figure 3. Photos taken of Ganoderma lucidum, Pleurotus ostreatus, and Trametes versicolor after 1, 4, and 7 days of incubation at 26 °C, 80% relative humidity, in the absence of light. Substrate IDs IV and V stand for mixture ratios of 1:3 and 0:4 of pine bark and cotton fiber, respectively.
The low growth on the first day of incubation can be explained by the lag phase in which the fungi adapt to the new growth conditions [Citation28]. They started to enter the log phase from the second day on, where they began to feed off the nutrients in the substrate to grow and expand their mycelium. The nutrients in the substrate were in excess, which enabled the extension of the mycelium to occur at a constant rate. The substrate type greatly influences mycelium growth speed since the hyphae directly interact with it and extract the essential nutrients [Citation29]. In general, investigated fungi performed better on mixtures rich in cotton fibers than those containing high amounts of pine bark.
shows the different growth rates of the fungi on substrates I–V, as well as the influence of CaCO3 addition and flush of CO2. The growth rate of G. lucidum was generally lowest on substrate I but increased as more cotton fibers were added to the mixture. The highest growth rates were achieved on substrate IV, which consists of 25% pine bark and 75% cellulose-rich cotton fiber. Substrates rich in fibers offer a unique structure promoting good absorption and high retention of water, which will stimulate better growth for the mycelium [Citation30]. This structure will help prevent the substrate from drying since it has a high water-holding capacity. The high radial expansion on substrates rich in fibers and the poor growth on substrates I and II can be explained by their low water-holding capacity. Thus, the mycelium dried out after a few days of incubation. By this, no further expansion was noticeable since dried-out samples stopped growing. However, if pure fiber substrate is used for cultivation, the growth rate decreases.
Figure 4. Radial expansion (mm d−1) of three Basidiomycota measured over seven days with 0, 5, and 10 vol.% flush of CO2 on the five different substrate mixtures with addition of 0, 2, and 5 wt.% CaCO3. Data were collected for three replicates of the experiment (n = 3) run in parallel. Radius was measured every 24 h in triplicate by the distance from the center of the inoculum to the longest visible hyphae.
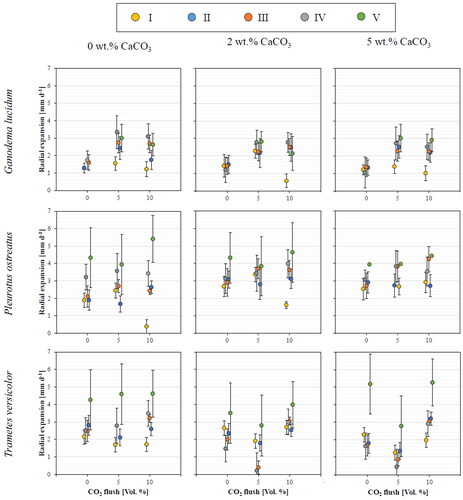
No positive effect on the mycelium growth of G. lucidum was observed concerning the addition of CaCO3. Although the initial pH level of the substrate should be higher than 5.5 [Citation31] and values of up to nine work best for G. lucidum [Citation20,Citation32], elevating it by using CaCO3 seems not improving the growth rate. Furthermore, the ideal initial pH depends on the fungal strain used within the same fungus species [Citation21]. In contrast, however, the flush of 5 vol.% CO2 promotes mycelium expansion on all five substrates – but the growth rate decreases at 10 vol.%. Consequently, the highest growth rate of G. lucidum of 3.34 mm d−m was determined on substrate IV without CaCO3 addition but flush of 5 vol.% CO2. Thus, adapted growth conditions in the incubator may lead to faster growth of G. lucidum and, therefore, shorten cultivation time.
Pleurotus ostreatus preferably degrades substrates rich in cellulose since a higher radial expansion of up to 4.33 mm d−m was detected the higher the cellulose content of the substrate mixture. According to this, substrate V performed best in all experimental series. Furthermore, flushing CO2 promotes mycelium formation, especially for substrates containing high proportions of cotton fibers. Thus, cultivation on substrate V and flushing with 10 vol.% CO2 led to the overall highest radial expansion rate of 5.40 mm d−1. Zadražil [Citation24] explains that a concentration of 17 vol.% CO2 in the environment stimulates the mycelium growth of the Pleurotus species and inhibits other nutrient-competing species, which might be within the substrate. Contamination was not visible for the samples from the P. ostreatus experiments. CaCO3 addition has a minor effect on the radial expansion of P. ostreatus cultivated on substrates III, IV, and V. Its mycelium can be cultivated at a broad pH range between 5 and 9. However, the highest growth rate was observed at 7 [Citation33]. Thus, substrate mixtures rich in pine bark offering a pH below 6, chemically elevating the pH value, can help the mycelium to establish faster. Besides the investigated factors, the growth of P. ostreatus can be enhanced by adapting the temperature in the incubator. Nashiruddin et al. [Citation28] investigated the mycelium expansion on rice husks at temperatures between 20 and 40 °C and stated that the best temperature is around 30 °C. However, Alam et al. [Citation33] reported that the maximum growth is recorded at 25 °C on potato dextrose agar. Therefore, the ideal environmental conditions for mycelium cultivation depend not only on the fungi but also on the substrate.
Trametes versicolor performed best on substrate V, reaching a radial expansion of up to 5.27 mm d−m. As no CaCO3 is added, a higher growth rate can be reached the higher the cotton fiber content in the substrate is. Elsacker et al. [Citation34] support this finding since they observed a white, dense T. versicolor mycelium layer on cellulose-rich substrates, such as hemp and flax. These biomasses offer high cellulose and low lignin contents of 70 and 5 wt.%, respectively. Further, they observed poor growth on pine softwood and straw with relatively high lignin contents of ca. 30 and 17 wt.%, respectively. These findings support the comparatively lowest growth rates of T. versicolor of 2.15, 1.69, and 1.71 mm d−m on substrate I with a flush of 0, 5, and 10 vol.% CO2, respectively. Further, the addition of 2 wt.% CaCO3 hinders mycelium formation, except for substrate I. Thus, chemically increasing the pH of acidic substrates can enhance the cultivation procedure. Flushing with 5 vol.% CO2 negatively impacts mycelium expansion, while 10 vol.% CO2 has a neglectable effect on the performance.
Although all substrates enabled the cultivation of the investigated fungi, the growth rate can be improved by adapting the N content. Attias et al. [Citation31] recommend N contents of the initial substrate with levels close to 1% for sufficient mycelium development based on their experiments on fungi from the Pleurotaceae family. Hence, increasing the nitrogen content by additives might enhance the suitability of biomass by narrowing the C/N ratio. Generally, ratios between 2 and 5 are recommended for T. versicolor, 1 for G. lucidum, and 18–38 for P. ostreatus cultivation [Citation20,Citation21, Citation35]. Potential nitrogen additives include yeast extract for enhanced mycelium expansion and density of T. versicolor [Citation21] and malt extract for G. lucidum cultivation [Citation20]. However, residues from food production might also serve as cheap nitrogen source, for instance, cassava [Citation36], wheat [Citation11, Citation22, Citation37], or rice bran [Citation38].
The good performance of all three investigated fungi on substrates rich in cotton fibers might be explained by its high content of K, which generally positively affects mycelium expansion [Citation35], wherefore additives such as K2HPO4 and KCl might promote mycelial growth [Citation20,Citation21]. Since the content of Zn was low in substrates with high cotton fiber content, the addition of Zn sulfate and Zn hydro aspartate may promote mycelial growth [Citation39]. In contrast to the other fungi, G. lucidum grew better on substrate IV and worse on substrate V. One reason for this may be the low Na content, which, however, can be added, e.g., as NaCl enhancing its mycelium formation [Citation20]. Regarding Mg availability, researchers found positive effects of media enrichment by adding the mineral salt MgSO4·7 H2O on the mycelial growth of T. versicolor and G. lucidum [Citation20,Citation21].
3.2. Mycelial density
As shown in , G. lucidum shows varying mycelial density dependent on CO2 flush and CaCO3 addition. Mycelium showed the most compact growth, cultivated on the substrates I, II, and III. Jo et al. [Citation20] stated that a higher mycelial density results from a narrow C/N ratio of the culture media. Hence, N additives such as calcium nitrate or malt extract might support denser mycelium formation [Citation20]. Mycelium grows less dense on the substrates with a pH >9. Thus, this study supports the findings that G. lucidum grows best up to that value [Citation20]. Other additives for pH elevation, such as NaOH, might be interesting to investigate since they led to higher colony diameter and denser mycelium in previous studies [Citation20].
Table 4. Effect of substrate, addition of buffer CaCO3 and flush of CO2 on the mycelial growth of three fungi, n = 3, mean ± std.
A dense growth of P. ostreatus was negatively affected by chemically elevated pH and flushing the incubator with CO2. Further, the fungus showed thin mycelium on substrates III–V. Hoa and Wang [Citation40] named sugarcane residues, acacia sawdust and corn straw as suitable lignocellulosic sources of P. ostreatus cultivation since the mycelium grew compact. On the other hand, sugarcane residues led to a lower mycelium colony of 54 mm in diameter after eight days compared to the radius of 53 mm measured after seven days of incubation in this study. In the future, a well-considered balance must be made between mycelium density and growth rate in mycelium composite production.
The same applies to T. versicolor, which primarily showed thin and somewhat thin mycelium density. Jo et al. [Citation21] found varying mycelium densities primarily dependent on the fungal strain and the C/N ratio of the medium. Here, narrow C/N ratio values lead to thinner growth. Thus, carbon sources, such as sucrose and mannitol, were found to be suitable to enhance mycelium density [Citation21].
4. Conclusions
Each fungus’ growth behavior primarily depends on the substrate composition, such as cellulose and lignin content, and its physical properties, i.e., the water-holding capacity, pH, C/N ratio, and content of macro- and micronutrients. Ganoderma lucidum showed the highest growth rate of 3.34 mm d−m on a mixture of 25 wt.% pine bark and 75 wt.% cotton fiber and a flush of 5 vol.% CO2. Cultivation of P. ostreatus on cotton fiber is positively affected by 10 vol.% CO2 in the environment, which led to a growth rate of 5.40 mm d−m.
Further, T. versicolor performed best on cotton fibers as well since it is rich in macronutrient K. All fungi preferred substrates with a high initial pH >6, but chemically elevating with CaCO3 lowered the growth rate or hindered dense mycelium formation. Further, the high water capacities of 89 and 90% for 25 wt.% pine bark with 75 wt.% cotton fiber and pure cotton fiber, respectively, led to sufficient growth during the seven days of incubation.
This method works to gain general insights into the growth behavior of mycelium on different substrate mixtures and under various growth conditions. However, identifying potential lignocellulosic residues and necessary additives to enhance their composition is crucial for sustainable mycelium cultivation.
Author contributions
K. A. Schoder: conceptualization, methodology, investigation, formal analysis, visualization, and writing – original draft preparation. J. Krümpel: conceptualization, data curation, writing supervision, and editing. A. Lemmer: methodology, writing, supervision – reviewing and editing. J. Müller: resources, supervision, writing – reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782. doi: 10.1126/sciadv.1700782.
- Majib NM, Sam ST, Yaacob ND, et al. Characterization of fungal foams from edible mushrooms using different agricultural wastes as substrates for packaging material. Polymers. 2023;15(4):873. doi: 10.3390/polym15040873.
- Atiwesh G, Mikhael A, Parrish CC, et al. Environmental impact of bioplastic use: a review. Heliyon. 2021;7(9):e07918. doi: 10.1016/j.heliyon.2021.e07918.
- Jiang L. A new manufacturing process for biocomposite sandwich parts using a myceliated core, natural reinforcement and infused bioresin. Ann Arbor, MI: ProQuest; 2015.
- Appels FVW, Camere S, Montalti M, et al. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater Des. 2019;161:64–71. doi: 10.1016/j.matdes.2018.11.027.
- Holt GA, McIntyre G, Flagg D, et al. Fungal mycelium and cotton plant materials in the manufacture of biodegradable molded packaging material: evaluation study of select blends of cotton byproducts. J Biobased Mater Bioenergy. 2012;6(4):431–439. doi: 10.1166/jbmb.2012.1241.
- Pelletier MG, Holt GA, Wanjura JD, et al. An evaluation study of mycelium based acoustic absorbers grown on agricultural by-product substrates. Ind Crops Prod. 2013;51:480–485. doi: 10.1016/j.indcrop.2013.09.008.
- Yang Z, Zhang F, Still B, et al. Physical and mechanical properties of fungal mycelium-based biofoam. J Mater Civil Eng. 2017;29(7):4017030. doi: 10.1061/(ASCE)MT.1943-5533.0001866.
- Jones M, Bhat T, Kandare E, et al. Thermal degradation and fire properties of fungal mycelium and mycelium–biomass composite materials. Sci Rep. 2018;8(1):17583. doi: 10.1038/s41598-018-36032-9.
- Tacer-Caba Z, Varis JJ, Lankinen P, et al. Comparison of novel fungal mycelia strains and sustainable growth substrates to produce humidity-resistant biocomposites. Mater Des. 2020;192:108728. doi: 10.1016/j.matdes.2020.108728.
- Sisti L, Gioia C, Totaro G, et al. Valorization of wheat bran agro-industrial byproduct as an upgrading filler for mycelium-based composite materials. Ind Crops Prod. 2021;170:113742. doi: 10.1016/j.indcrop.2021.113742.
- Kameshwar AKS, Qin W. Lignin degrading fungal enzymes. In: Fang Z, Smith RL, editors. Production of biofuels and chemicals from lignin. Singapore: Springer Singapore (Biofuels and Biorefineries); 2016. p. 81–130.
- Floudas D, Binder M, Riley R, et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336(6089):1715–1719. doi: 10.1126/science.1221748.
- Bennet JW, Wunch KG, Faison BD. Use of fungi biodegradation. In: Manual of environmental microbiology [cited 2024 Jan 16]; 2002. p. 960–971. Available from: https://nishat2013.files.wordpress.com/2013/11/fungi-biodegradation-book.pdf
- Enoki A, Tanaka H, Fuse G. Degradation of lignin-related compounds, pure cellulose, and wood components by white-rot and brown-rot fungi. Holzforschung. 1988;42(2):85–93. doi: 10.1515/hfsg.1988.42.2.85.
- Have RT, Teunissen PJ. Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem Rev. 2001;101(11):3397–3413. doi: 10.1021/cr000115l.
- Jones M-P, Lawrie AC, Huynh TT, et al. Agricultural by-product suitability for the production of chitinous composites and nanofibers utilising Trametes versicolor and Polyporus brumalis mycelial growth. Process Biochem. 2019;80:95–102. doi: 10.1016/j.procbio.2019.01.018.
- Xing Y, Brewer M, El-Gharabawy H, et al. Growing and testing mycelium bricks as building insulation materials. IOP Conf Ser Earth Environ Sci. 2018;121:22032. doi: 10.1088/1755-1315/121/2/022032.
- Cerimi K, Akkaya KC, Pohl C, et al. Fungi as source for new bio-based materials: a patent review. Fungal Biol Biotechnol. 2019;6:17. doi: 10.1186/s40694-019-0080-y.
- Jo W-S, Cho Y-J, Cho D-H, et al. Culture conditions for the mycelial growth of Ganoderma applanatum. Mycobiology. 2009;37(2):94–102. doi: 10.4489/MYCO.2009.37.2.094.
- Jo W-S, Kang M-J, Choi S-Y, et al. Culture conditions for mycelial growth of Coriolus versicolor. Mycobiology. 2010;38(3):195–202. doi: 10.4489/MYCO.2010.38.3.195.
- Schritt H, Vidi S, Pleissner D. Spent mushroom substrate and sawdust to produce mycelium-based thermal insulation composites. J Clean Prod. 2021;313:127910. doi: 10.1016/j.jclepro.2021.127910.
- Tudryn GJ, Smith LC, Freitag J, et al. Processing and morphology impacts on mechanical properties of fungal based biopolymer composites. J Polym Environ. 2018;26(4):1473–1483. doi: 10.1007/s10924-017-1047-9.
- Zadražil F. Influence of CO2 concentration on the mycelium growth of three Pleurotus species. Eur J Appl Microbiol. 1975;1(4):327–335. doi: 10.1007/BF01382692.
- Haneef M, Ceseracciu L, Canale C, et al. Advanced materials from fungal mycelium: fabrication and tuning of physical properties. Sci Rep. 2017;7:41292. doi: 10.1038/srep41292.
- Ziegler AR, Bajwa SG, Holt GA, et al. Evaluation of physico-mechanical properties of mycelium reinforced green biocomposites made from cellulosic fibers. Appl Eng Agric. 2016;32(6):931–938. doi: 10.13031/aea.32.11830.
- Alemu D, Tafesse M, Gudetta Deressa Y. Production of mycoblock from the mycelium of the fungus Pleurotus ostreatus for use as sustainable construction materials. Adv Mater Sci Eng. 2022;2022:1–12. doi: 10.1155/2022/2876643.
- Nashiruddin NI, Chua KS, Mansor AF, et al. Effect of growth factors on the production of mycelium-based biofoam. Clean Technol Environ Policy. 2022;24(1):351–361. doi: 10.1007/s10098-021-02146-4.
- Bellettini MB, Fiorda FA, Maieves HA, et al. Factors affecting mushroom Pleurotus spp. Saudi J Biol Sci. 2019;26(4):633–646. doi: 10.1016/j.sjbs.2016.12.005.
- Siwulski M, Drzewiecka K, Sobieralski K, et al. Comparison of growth and enzymatic activity of mycelium and yielding of Pleurotus ostreatus (Fr.) Kumm. on different substrates. Acta Sci Polon Hort Cultus. 2010;9(3):45–50.
- Attias N, Danai O, Ezov N, et al. Developing novel applications of mycelium based bio-composite materials for design and architecture. In: Attias N, Danai O, Ezov N, et al. Building with Bio-Based Materials: Best Practice and Performance Specification; Zagreb, Croatia: Faculty of Forestry, University of Zagreb; 2017.
- Jayasinghe C, Imtiaj A, Hur H, et al. Favorable culture conditions for mycelial growth of Korean wild strains in Ganoderma lucidum. Mycobiology. 2008;36(1):28–33. doi: 10.4489/MYCO.2008.36.1.028.
- Alam N, Lee J, Lee T. Mycelial growth conditions and phylogenetic relationships of Pleurotus ostreatus. World Appl Sci J. 2010;9(8):928–937.
- Elsacker E, Vandelook S, Brancart J, et al. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLOS One. 2019;14(7):e0213954. doi: 10.1371/journal.pone.0213954.
- Koutrotsios G, Mountzouris KC, Chatzipavlidis I, et al. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi – assessment of their effect on the final product and spent substrate properties. Food Chem. 2014;161:127–135. doi: 10.1016/j.foodchem.2014.03.121.
- Lima GGd, Schoenherr ZCP, Magalhães WLE, et al. Enzymatic activities and analysis of a mycelium-based composite formation using peach palm (Bactris gasipaes) residues on Lentinula edodes. Bioresour Bioprocess. 2020;7(1). doi: 10.1186/s40643-020-00346-2.
- Matos MP, Teixeira JL, Nascimento BL, et al. Production of biocomposites from the reuse of coconut powder colonized by shiitake mushroom. Ciênc Agrotec. 2019;43:e003819. doi: 10.1590/1413-7054201943003819.
- Rocha MI, Benkendorf S, Gern RM, et al. Desenvolvimento de biocompósitos fúngicos utilizando resíduos industriais. Matéria. 2020;25(4):e12840. doi: 10.1590/s1517-707620200004.1140.
- Zięba P, Kała K, Włodarczyk A, et al. Selenium and zinc biofortification of Pleurotus eryngii mycelium and fruiting bodies as a tool for controlling their biological activity. Molecules. 2020;25(4):889. doi: 10.3390/molecules25040889.
- Hoa HT, Wang C-L. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 2015;43(1):14–23. doi: 10.5941/MYCO.2015.43.1.14.

