ABSTRACT
Malignant pleural mesothelioma is a rare and fatal malignancy. This disease is, unfortunately, at its advanced stage when it is diagnosed. Survival time is usually not more than a few months. The aim of this study was to analyse the expression of Aquaporin 1, Aquaporin 3 and Aquaporin 5 in malignant pleural mesotheliomas and to explore the relationship of these levels of expression with epidermal growth factor receptor (EGFR) gene mutation and prognostic parameters. In this study, 60 cases diagnosed as malignant pleural mesothelioma among the pleural biopsy materials in the archives of the Pathology Department of Medical Faculty of Dicle University in 2003–2013 were evaluated. The tissues were stained immunohistochemically with antibodies against Aquaporin 1, Aquaporin 3 and Aquaporin 5, and the existence of EGFR mutation was investigated in the tissues by real-time polymerase chain reaction (PCR). The obtained results showed expression of Aquaporin 1, Aquaporin 3 and Aquaporin 5 in varied amounts in malignant pleural mesotheliomas. However, no significant relation was obtained thus far between the expression levels of these aquaporins and the prognostic parameters. No mutations were detected in the EGFR gene exons 18–21 by using real-time PCR. It could be suggested that although Aquaporin 1, Aquaporin 3 and Aquaporin 5 are expressed in malignant pleural mesothelioma, they do not have any effect on the prognostic parameters. Mutations in different domains of EGFR gene, other than exons 18–21, should be sought to develop new targeted treatments.
Introduction
Mesothelioma is a rare and deadly malignancy. This disease has been associated with occupational and environmental asbestos exposure [Citation1]. Mesothelioma is often at its advanced stage when it is diagnosed. Survival time is usually not more than a few months. Several therapeutic approaches such as surgery, radiation, surgery combined with radiation, and chemotherapy in various combinations with radiotherapy and surgery have been tried to extend the survival time [Citation2–4].
Aquaporins (AQPs) are a class of widespread small integral membrane proteins, with 13 members of them (AQP0–12) identified in mammals [Citation5–7]. Recently, AQPs have been determined to play significant roles in tumour pathogenesis. Since AQPs are involved in cell migration and proliferation, they are considered part of an ever growing list of effectors in tumour biology [Citation8–10]. Earlier studies have shown that AQP1, AQP3 and AQP5 are highly expressed in colorectal, lung carcinomas and gliomas [Citation10,Citation11].
Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor which plays an important role in transferring extracellular signals from the cell surface to the cell interior. Some of the critical processes which EGFR mediates include cell proliferation, differentiation, migration and apoptosis. Dysregulated expression of tyrosine kinase receptors may cause aberrations of homeostatic cellular processes, eventuating in malignant transformation of cells. EGFR mutations are detected in cancers like head and neck cancers and non-small-cell lung cancers. These mutations are indicators that patients will respond to gefitinib or erlotinib therapy [Citation12–14]. Some studies have shown that immunohistochemical overexpression of the EGFR protein takes place in malignant pleural mesotheliomas (MPMs) [Citation15,Citation16].
The aim of this study was to analyse the expression levels of AQP1, AQP3 and AQP5 in MPMs, to investigate the relationship between these expression levels and prognostic parameters and to screen for mutations in EGFR exons 18–21 in order to predict weather target treatment would succeed or not.
Materials and methods
Tissue collection and examination
In this study, we evaluated 60 MPM cases diagnosed among the pleural biopsy materials in the archives of the Pathology Department of Medical Faculty of Dicle University in the period 2003–2013. We worked over all the cases by light microscope (Olympus BX53, Tokyo, Japan) and subtyped them histopathologically (epithelioid, sarcomatoid, desmoplastic and biphasic) according to the World Health Organization classification guidelines [Citation17]. We documented the clinical records and histopathological diagnosis of all patients. All the cases were staged clinically according to the tumor, node, metastasis (TNM) classification of pleural mesothelioma [Citation18]. Local ethical approval was obtained from the Dicle University Medical Faculty Ethics Committee.
Immunohistochemical assay
Four-micrometre sections were prepared from routinely processed paraffin blocks. The sections were mounted on positively charged slides and were incubated at 57 °C for 60 minutes to remove the paraffin. Immunohistochemical staining was performed with the automated BenchMark XT immunohistochemical system (Ventana Medical Systems, Tucson, AZ, USA). Briefly, AQP1, AQP3 and AQP5 were detected by using monoclonal anti-AQP1 antibodies (ab9566, Abcam, Cambridge, UK), anti-AQP3 antibodies (ab125219, Abcam) and anti-AQP5 antibodies (ab134687, Abcam).
Immunohistochemical staining assessment was modelled from the study by Özler et al. [Citation19]. The staining intensity and extent of stained cells were evaluated and scored for each sample. The distribution of AQP1, AQP3 and AQP5 immunoreactivity was semi-quantitatively scored by using a 0--4 scale for the percentage of stained cells. A score of 0 represented none to <5% of cells stained, 1+ was 6%–25% of cells stained, 2+ was 26%–50% of cells stained, 3+ was 51%–75% of cells stained and 4+ was 76%–100% of cells stained. The immunohistochemical staining intensity was graded from 0 to 3, with 0 being none, 1 being weak, 2 being moderate and 3 being strong. The combined scores were calculated as a sum of the extent and intensity scores. Finally, the combined scores were graded as: negative (0) = 0, weak (1) = 1 or 2, moderate (2) = 3 or 4 and strong (3) = 5–7.
The evaluation criteria were as follows: age (≤60 or >60 years), gender, histopathological subtype (epithelial or non-epithelial), stage of the disease (stage 1–2 or stage 3–4), low (score of 1 or 2) or high (score of 3) AQP1 expression, low (score of 1 or 2) or high (score of 3) AQP3, low (score of 1) or high (score of 2) AQP5 expression.
EGFR mutation
The cobas® EGFR Mutation Test kit (AS-PCR test, Roche Diagnostics GmBH, Mannheim, Germany) is a Conformité Européene – in-vitro diagnostic (CE-IVD)-marked allele-specific polymerase chain reaction (PCR) test designed to detect the presence of 41 mutations in exons 18, 19, 20 and 21 of the EGFR gene in MPMs formalin-fixed paraffin-embedded tissue specimens. The test requires 150 nanogram total DNA input, which can typically be obtained by using one 5 μm formalin-fixed paraffin-embedded tissue section. Positive and negative controls were used in all tests. All analyses and reports of the results were fully automated.
Statistical analysis
SPSS version 18.0 (Statistical Package for the Social Sciences) was used for statistical analysis. The correlation between AQP1, AQP3, AQP5 expressions and the clinical and pathological characteristics of the patients was analysed by using the Chi-square test. The effects of AQP1, AQP3, AQP5 expressions, clinical and pathological characteristics on general survival time were evaluated by the Kaplan–Meier method in univariate analysis and by the Cox regression method in multivariate analysis. The results were considered statistically significant when the p value was less than 0.05 (p < 0.05).
Results and discussion
Cohort of patients
In this study, we explored the potential association between the expression levels of AQP1, AQP3 and AQP5 in MPMs with prognostic parameters and screened for mutations in EGFR exons 18–21 as predictors for the success of target treatment. The study cohort included 60 MPM patients, 20 of whom were women and 40 were men. The average age of the patients was 61.28 ± 11.26 years. Histological examination showed that 50 MPMs (83.3%) were epithelioid, 6 (10%) were sarcomatoid and 4 (6.7%) were biphasic (). Despite treatment, the average survival time of patients with MPM is 12 months from the time of diagnosis [Citation20]. In our study, at the time of diagnosis, 3 (5%) of the patients were stage 2, 13 (21.67%) of them were stage 3 and 44 (73.33%) were stage 4. The survival time of the patients varied between 1 and 50 months and the average survival time was 11.68 ± 9.72 months. There was a statistically significant relation between the stage and the survival time (p < 0.05). The clinical and the prognostic features as well as the results from the EGFR mutation analysis and from the immunohistochemical staining are shown in
Table 1. Clinical and prognostic features of the patients and results from the EGFR mutation analysis and immunohistochemical staining.
Aquaporin levels
As a first step, we performed immunohistochemical analysis of the levels of AQPs in the studied MPMs, since AQPs have been reported to be expressed in many tumour cells in humans and rodents. For some tumours, there are positive correlations between histological tumour grade and the amount of AQP expression [Citation10]. AQPs are a family of membrane transport proteins which are small (25–34 kDa) and hydrophobic. As reviewed by Verkman et al. [Citation10], they assemble in membranes as tetramers and act primarily as water-selective pores that facilitate osmotically driven water transport across cell plasma membranes [Citation5].
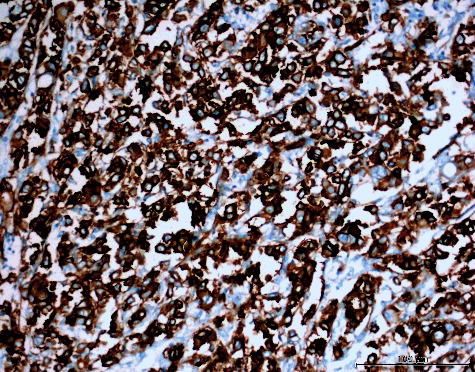
AQP1 is abundant in the endothelial cells of microvessels and is also expressed in secreting and absorbing epithelia. Its main function is to increase the osmotic-driven transport of water across cell membranes [Citation21]. AQP1 is expressed in the microvessels of various types of tumours, suggesting that it could possibly be involved in tumour angiogenesis [Citation22]. AQP1 expression has been reported to arise in lung adenocarcinoma, hemangioblastoma, choroid plexus tumours, cholangiocarcinoma, glioma and laryngeal cancer (reviewed in [Citation10]). In our study, we detected strong membranous and cytoplasmic staining for AQP1 in 50 (83.33%) cases, moderate staining in 8 (13.33%) cases and weak staining in 1 (1.67%) case (). In one (1.67%) case, no staining with AQP1 was observed. Statistically, no significant relation was determined between the AQP1 expression level and the MPMs histological type (p > 0.05); therefore, strong AQP1 expression levels would be observed in MPMs regardless of the histological subtype. Kao et al. [Citation23] have reported that AQP1 is an independent prognostic factor in MPMs. Unlike their observation, in our study, there was no statistically significant relation between AQP1 expression and the age, sex and survival time of the patients or with the stage of the disease (p > 0.05).
Figure 1. Diffuse (extensity score = 4) and strong (intensity score = 3) expression of AQP1 in MPM (AQP1 200×).
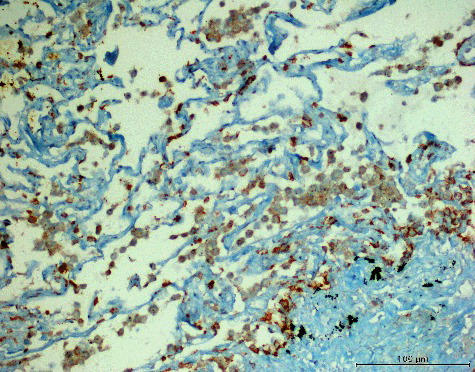
AQP3 plays an important role in fluid homeostasis and has been observed to be expressed in many carcinomas [Citation24–32]. In our study, the immunohistochemical analysis revealed strong membranous and cytoplasmic staining for AQP3 in 5 (8.33%) cases, moderate staining in 26 (43.33%) cases () and weak staining in 7 (11.67%) cases. No AQP3 expression was observed in 22 (36.67%) cases. The statistical analysis did not reveal significant relation between the AQP3 expression level and MPMs histological type (p > 0.05). Furthermore, the AQP3 expression level was not statistically significantly associated either with the age, sex and survival time of the patients, or with the stage of the disease (p > 0.05). Since more than half of the cases showed AQP3 expression in variable amounts, it is natural to say that AQP3 expression might be observed in MPMs, but this expression has no effect on the survival time or the stage of the disease.
Figure 2. Focal (extensity score = 2) and moderate (intensity score = 2) expression of AQP3 in MPM (AQP3, 200×).
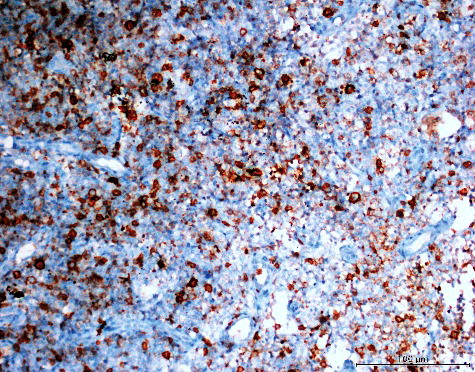
AQP5 is a protein expressed in many ephitelia, including the salivary and lacrimal gland, and plays an important role in the water movement for creation of saliva, tears, etc. [Citation33,Citation34]. AQP5 is presumed to be related to tumourigenesis and tumour progression of various cancers, including cancers of the lung [Citation35], colorectal [Citation36], gastric [Citation37], ovary cancer [Citation38] and gliomas [Citation39]. In our study, there was moderate membranous and cytoplasmic immunohistochemical staining for AQP5 in 12 (20%) cases and weak staining in 11 (18.33%) cases. (), whereas no AQP5 staining was observed in 37 (61.67%) cases. Strong staining for AQP5 was obtained in none of the studied cases. There was again no statistically significant relation between AQP5 expression and the MPMs histological type, age, sex, survival time of the patients or the stage of the disease (p > 0.05). Since weak/moderate AQP5 expression was observed in less than half of the cases, it could be suggested that AQP5 may be expressed in MPMs, but not as frequently as AQP1 and AQP3. In addition, AQP5 expression had no relation with the prognostic parameters, similar to AQP1 and AQP3 expression. There are some reports that AQP1, AQP3 and AQP5 expression levels increase together in colorectal cancer [Citation40], lung cancer [Citation11], gastric cancer [Citation41] and gliomas [Citation10]. In line with these reports, in our study, we also observed increased AQP1, AQP3 and AQP5 expression levels; however, our results showed that these expression levels could not be used as a prognostic indicator.
EGFR mutation analysis
EGFR is arising as an important target for therapy of various malignancies. The inhibition of the EGFR pathways causes an anti-tumour effect. For cancer therapy, two classes of EGFR inhibitors have been developed. These classes are tyrosine kinase inhibitors (TKIs) and anti-EGFR monoclonal antibodies. In several studies, EGFR expression has been reported immunohistochemically in 32%–68% of MPMs [Citation15,Citation42–44]. EGFR mutations have been detected by fluorescent in situ hybridization in 3%–4% of MPMs [Citation16,Citation44]. There are also some studies that have made use of direct sequencing, which is a less sensitive method, to search for mutations in the tyrosine kinase domain of the EGFR gene but these studies deal with only small populations of MPM patients [Citation45–47]. Mezzapelle et al. [Citation48] have detected no mutation in the tyrosine kinase domain of EGFR in MPM cases by using PCR. Although in a preclinical study, Barbieri et al. [Citation49] showed effectiveness of EGFR TKIs, Govindan et al. [Citation50] and Garland et al. [Citation51] reported that gefitinib and erlotinib have no clinical efficacy in their phase II studies. In our study, we detected no mutations in EGFR gene exons 18–21 in 60 MPM cases by using real-time PCR. This might be one potential reason why the TKIs fail in the treatment of MPMs, suggesting that patients may be directed towards new targeted treatments.
Conclusions
In this study, AQP1, AQP3 and AQP5 showed varied expression levels in MPMs. However, these expression levels were not observed to be associated with the prognostic parameters. Further studies are required to show if immunohistochemical markers can be used in differential diagnosis of MPMs from other tumours. The absence of mutations in EGFR exons 18–21 in the 60 MPM cases analysed by real-time PCR suggests that mutations in different domains of the EGFR gene should be sought to produce new targeted treatments.
Acknowledgments
We thank Dicle University Scientific Research Projects Coordination Unit(DUBAP) for the financial support through grant number:14-TF-56.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Critical Rev Toxicol. 2009;39:576–588.
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomized feasibility study. Lancet Oncol. 2011;12:763–772.
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of survival in malignant pleural mesothelioma: a surveillance, epidemiology, and end results (SEER) study of 14,228 patients. PLoS One. [Internet]. 2015 [ cited 2016 Nov 19];10:e0145039. Available from: http://journals.plos.org/plosone/article?id = 10.1371/journal.pone.0145039
- Stahel RA, Weder W, Felley-Bosco E, et al. Searching for targets for the systemic therapy of mesothelioma. Ann Oncol. 2015;26:1649–1660.
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278:13–28.
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698.
- Huang YT, Zhou J, Shi S, et al. Identification of estrogen response element in Aquaporin-3 gene that mediates estrogen-induced cell migration and ınvasion in estrogen receptor-positive breast cancer. Sci Rep. [Internet]. 2015 [ cited 2016 Nov 19];5:12484. Available from: http://www.nature.com/articles/srep12484
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, et al. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792.
- Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–1894.
- Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins – new players in cancer biology. J Mol Med (Berl). 2008;86:523–529.
- Machida Y, Ueda Y, Shimasaki M, et al. Relationship of aquaporin 1, 3, and 5 expression in lung cancer cells to cellular differentiation, invasive growth, and metastasis potential. Hum Pathol. 2011;42:669–678.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139.
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500.
- Lee JW, Soung YH, Kim SY, et al. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:2879–2882.
- Rena O, Boldorini LR, Gaudino E, et al. Epidermal growth factor receptor overexpression in malignant pleural mesothelioma: prognostic correlations. J Surg Oncol. 2011;104:701–705.
- Enomoto Y, Kasai T, Takeda M, et al. A comparison of epidermal growth factor receptor expression in malignant peritoneal and pleural mesothelioma. Pathol Int. 2012;62:226–231.
- Galateau-Salle F, Churg A, Roggli V, et al. The 2015 World Health Organization Classification of Tumors of the Pleura: advances since the 2004 classification. J Thorac Oncol. 2016;11:142–154.
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479–495.
- Özler A, Evsen MS, Turgut A, et al. CD147 expression in uterine smooth muscle tumors, and its potential role as a diagnostic and prognostic marker in patients with leiomyosarcoma. J Exp Ther Oncol. 2014;10:325–330.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644.
- Matsuzaki T, Tajika Y, Ablimit A, et al. Aquaporins in the digestive system. Med Electron Microsc. 2004;37:71–80.
- Mobasheri A, Airley R, Hewitt SM, et al. Heterogeneous expression of the aquaporin 1 (AQP 1) water channel in tumors of the prostate, breast, ovary, colon and lung: a study using high density multiple human tumor tissue microarrays. Int J Oncol. 2005;26:1149–1158.
- Kao SC, Armstrong N, Condon B, et al. Aquaporin 1 is an independent prognostic factor in pleural malignant mesothelioma. Cancer. 2012;118:2952–2961.
- Litman T, Sogaard R, Zeuthen T. Ammonia and urea permeability of mammalian aquaporins. Handb Exp Pharmacol. 2009;190:327–358.
- Wang G, Gao F, Zhang W, et al. Involvement of aquaporin 3 in Helicobacter pylori-related gastric diseases. PLoS One. [ Internet]. 2012 [ cited 2016 Aug 23];7:e49104. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0049104
- Kusayama M, Wada K, Nagata M, et al. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. 2011;102:1128–1136.
- Shi YH, Chen R, Talafu T, et al. Significance and expression of aquaporin 1, 3, 8 in cervical carcinoma in Xinjiang Uygur women of China. Asian Pac J Cancer Prev. 2012;13:1971–1975.
- Niu D, Kondo T, Nakazawa T, et al. Differential expression of aquaporins and its diagnostic utility in thyroid cancer. PLoS One. [Internet]. 2012 [ cited 2016 Aug 23];7:e40770. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0040770
- Liu W, Wang K, Gong K, et al. Epidermal growth factor enhances MPC-83 pancreatic cancer cell migration through the up-regulation of aquaporin 3. Mol Med Rep. 2012;6:607–610.
- Ismail M, Bokaee S, Morgan R, et al. Inhibition of the aquaporin 3 water channel increases the sensitivity of prostate cancer cells to cryotherapy. Br J Cancer. 2009;100:1889–1895.
- Ji C, Cao C, Lu S, et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother Pharmacol. 2008;62:857–865.
- Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–332.
- Janosi L, Ceccarelli M. The gating mechanism of the human aquaporin 5 revealed by molecular dynamics simulations. PLoS One. [Internet]. 2013 [ cited 2016 Aug 23];8:e59897. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0059897
- Matsuzaki T, Susa T, Shimizu K, et al. Function of the membrane water channel aquaporin-5 in the salivary gland. Acta Histochem Cytochem. 2012;45:251–259.
- Zhang Z, Chen Z, Song Y, et al. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J Pathol. 2010;221:210–220.
- Kang SK, Chae YK, Woo J, et al. Role of human aquaporin 5 in colorectal carcinogenesis. Am J Pathol. 2008;173:518–525.
- Watanabe T, Fujii T, Oya T, et al. Involvement of aquaporin-5 in differentiation of human gastric cancer cells. J Physiol Sci. 2009;59:113–122.
- Yang JH, Shi YF, Cheng Q, et al. Expression and localization of aquaporin-5 in the epithelial ovarian tumors. Gynecol Oncol. 2006;100:294–299.
- Boon K, Edwards JB, Eberhart CG, et al. Identification of astrocytoma associated genes including cell surface markers. BMC Cancer. [Internet]. 2004 [ cited 2016 Aug 23];4:39. Available from: http://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-4-39
- Moon C, Soria JC, Jang SJ, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–6703.
- Shen L, Zhu Z, Huang Y, et al. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother. 2010;64:313–318.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated nonsmall-cell lung cancer. N Engl J Med. 2005;353:123–132.
- Dazzi H, Hasleton PS, Thatcher N, et al. Malignant pleural mesothelioma and epidermal growth factor receptor (EGFR). Relationship of EGFR with histology and survival using fixed paraffin-embedded tissue and the F4, monoclonal antibody. Br J Cancer. 1990;61:924–926.
- Okuda K, Sasaki H, Dumontet C, et al. Epidermal growth factor receptor gene mutation, amplification and protein expression in malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2008;134:1105–1111.
- Cortese JF, Gowda AL, Wali A, et al. Common EGFR mutations conferring sensitivity to gefitinib in lung adenocarcinoma are not prevalent in human malignant mesothelioma. Int J Cancer. 2006;118:521–522.
- Velcheti V, Kasai Y, Viswanathan AK, et al. Absence of mutations in the epidermal growth factor receptor (EGFR) kinase domain in patients with mesothelioma. J Thorac Oncol. 2009;4:559.
- Enomoto Y, Kasai T, Takeda M, et al. Epidermal growth factor receptor mutations in malignant pleural and peritoneal mesothelioma. J Clin Pathol. 2012;65:522–527.
- Mezzapelle R, Miglio U, Rena O, et al. Mutation analysis of the EGFR gene and downstream signalling pathway in histologic samples of malignant pleural mesothelioma. Br J Cancer. 2013;108:1743–1749.
- Barbieri F, Wurth R, Favoni RE, et al. Receptor tyrosine kinase inhibitors and cytotoxic drugs affect pleural mesothelioma cell proliferation: insight into EGFR and ERK1/2 as antitumor targets. Biochem Pharmacol. 2011;82:1467–1477.
- Govindan R, Kratzke RA, Herndon JE, et al. Gefitinib in patients with malignant mesothelioma: a phase II study by the cancer and leukemia group B. Clin Cancer Res. 2005;11:2300–2304.
- Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol. 2007;25:2406–2413.