ABSTRACT
This study examined osmotic adjustment (OA) and solutes accumulated in leaf segments of wheat cultivar Hartog exposed to polyethylene glycol (PEG)-induced water deficit (WD) in laboratory experiments. Additional 10 mM K+ or Na+ added to basal solution with PEG −0.5 MPa enhanced leaf OA by up to 100%. Omission of nitrogen from the basal solution suppressed the expression of OA in leaf segments, but the addition of 10 mM NO3– increased leaf OA by up to 81%. K+ and glycinebetaine were not accumulated in leaf segments exposed to WD under PEG −0.5 MPa during 48 h, but Na+, Cl−, proline and total soluble sugars accounted for up to 21, 20, 7 and 40% of OA, respectively. Total soluble sugars were the largest contributor to OA and may also contribute to membrane stability. Further data are needed on accumulation of leaf NO3− and glycinebetaine in response to increasing nitrogen supply, and on which other inorganic and organic solutes may also contribute to OA under PEG-induced WD. Laboratory experiments with leaf segments exposed to WD under −0.5 MPa PEG, and with 10 mM K+, Na+ or NO3− added to the basal solution, could provide an effective and rapid pre-screen of diverse germplasm sources for OA expression. Selected genotypes should then be validated by exposure to soil WD for agronomic evaluation, and for OA expression under field WD.
1. Introduction
Water deficit (WD) is common in dryland wheat farming, and is the major abiotic factor limiting crop productivity (Blum, Citation1988), so understanding is needed of the physiological, genetic and biochemical mechanisms of WD tolerance for crop improvement. One important component of plant WD tolerance is osmotic adjustment (OA) (Jones et al., Citation1981), which is the lowering of osmotic potential (OP) in cells caused by the net accumulation of solutes in response to WD (Zhang et al., Citation1999), allowing plants to maintain turgor at low tissue water potential (Jones et al., Citation1981).
Polyethylene-glycol (PEG) of high molecular weight has been used in controlled-environment experiments as a non-permeating osmotic agent to lower the external water potential to induce WD in plants (Bressan et al., Citation1981). PEG 6000 applied to roots of 3-month-old pepper plants (Capsicum frutescens L.) in half-strength Hoagland’s solution plus 0.5 mM NaCl, resulted in changes in plant water relations similar to those caused by drying soil at the same water potential (Kaufmann & Eckard, Citation1971).
Detached leaves, including leaf discs and leaf segments, have been used as experimental models to investigate physiological changes in leaf tissues of several plant species subjected to in vitro PEG-induced WD. Lascano et al. (Citation2001) reported that antioxidant enzymes (superoxide dismutase, ascorbate peroxidase, glutathione reductase) that can mitigate oxidative damage were induced in leaf segments of drought-tolerant wheat subjected for 48 h to −1.5 MPa PEG 8000. Hsu and Kao (Citation2003) treated detached 3rd leaves of 12-d-old rice (Oryza sativa L.) seedlings with PEG 6000 (−1.5 MPa) for 12 h and reported decrease in relative water content (RWC), protein, chlorophyll, and increase in proline.
Different rates of imposing WD using PEG solution resulted in different characteristics of solute accumulation. Naidu et al. (Citation1990) used PEG to induce WD in wheat seedlings at a slow (at −0.3 MPa d−1 to a final level of −1.5 MPa) and rapid rate (−1.5 MPa ‘shock’). Proline concentration in leaves with the slower rate of PEG application (120 μmol g−1 DM) was 1.7 times higher than that with the ‘shock’ treatment. Glycinebetaine concentration in leaves with the slower rate (44 μmol g−1 DM) was 3-times higher than when PEG ‘shock’ was imposed (Naidu et al., Citation1990). Nevertheless, in both types of treatments (slow and shock PEG), proline concentration and glycinebetaine concentration were much higher than those in the control (0.2 and 6.7 μmol g−1 DM, respectively). Proline concentration and glycinebetaine concentration with incremental PEG application were higher than at an abrupt osmotic ‘shock’, which is often imposed in many laboratory experiments using PEG.
Kocheva et al. (Citation2007) compared solutes accumulated under an osmotic potential of −1.2 MPa (25% PEG 8000) in barley (Hordeum vulgare L.) seedlings in 25% and 100% Knop nutrient solution, that, respectively, contained 2 mM and 8 mM nitrogen (N as NO3−). After 48 h of treatment, proline concentration in leaf tissues (500 μmol g−1 DM) in 100% Knop solution was 60% greater than in the 25% Knop solution, whereas the concentration of reducing sugars (225 μmol g−1 DM) was 50% higher in leaf tissues in 25% Knop solution. The authors concluded that the availability of N might have altered the synthesis of N-containing solutes.
Nio et al. (Citation2011) examined expression of OA and accumulation of solutes in leaves of wheat (Triticum aestivum L.) cultivars Hartog and Sunco in response to WD imposed at reproductive stage, in intact plants grown in 0.80 m soil columns in a controlled environment. Leaf OA was 5-times greater in Hartog, which also accumulated dry matter (DM) faster with greater soil water extraction than in Sunco. Leaf OA increased to 0.37 MPa at 37 d of withholding water. K+ was the major contributor to OA (viz. 54%) up to 30 d of drying, whereas glycinebetaine, proline and glucose were major contributors later (19, 21 and 22% of OA at 37 d, respectively). Thus, the solutes that contributed to leaf OA accumulated at different times as WD developed.
Nio et al. (Citation2018) examined OA expression and solutes involved in leaves of wheat cultivar Hartog under WD induced at tissue level using PEG8000 as a non-permeating osmotic agent in the laboratory. Exposure of leaf segments to PEG8000 treatments of 0, −0.5, −1.0 and −1.5 MPa and sampling times of 0, 12, 24, 48 and 72 h showed that maximum leaf OA (0.37 MPa) was expressed on PEG −0.5 MPa after 48 h of treatment. K+ did not contribute to OA, while Na+ and proline only accounted for 5 and 1%. Nio et al. (Citation2018) concluded that further experiments were needed to measure other solutes that contributed to leaf OA at tissue level under PEG-induced WD. This paper reports those subsequent experiments.
The literature above shows that responses to PEG may vary with conditions of treatment, but some aspects of tissue-level responses to WD can be studied in vitro, using PEG. Nevertheless, care should be taken in using PEG as an osmotic agent to induce WD in plants, such as using high molecular weight PEG (6000 to 8000), aerating the solution, and avoiding any root damage (Blum, Citation1998). We took these precautions in the following experiments.
The overall purpose was to clarify the effects of PEG-induced WD on the OA expression and solute concentrations in leaf segments of wheat, for potential pre-screening of germplasm for OA capacity. Such laboratory experiments should save time and money, allowing much larger and more diverse germplasm collections to be pre-screened. Consequently, this study evaluates OA expression and solutes accumulated in leaf segments of wheat exposed to WD induced by −0.5 MPa PEG 8000. The hypotheses were that nitrogen was essential in basal solution, that additional solutes added to basal solution would further enhance OA expression, and if OA expression did increase, that PEG-induced WD may be suitable for rapid germplasm pre-screening for OA expression. The objectives were to evaluate the effects of additional K+ and Na+, and the effects of N supply, on OA expression and the accumulation of inorganic and organic solutes in wheat leaf segments at 48 h of treatment. The potential to use PEG-induced WD for rapid pre-screening of diverse germplasm sources for OA expression is discussed.
2. Materials and methods
2.1 General procedures
Wheat was used in two experiments with segments of fully-expanded flag leaves (FEFL). Hartog is a wheat cultivar with high OA capacity (Nio et al., Citation2011). The plants were grown in containers (12 L) in a controlled-environment room with 10 h day and 14 h night at 19/14°C, relative humidity was 70%, and the average photosynthetically active radiation at plant height was 460 µmol quanta m−2 s−1.
Leaf blades of FEFL were detached from 81 d-old-plants, i.e. in the middle part of linear phase of grain growth. Leaves were rinsed with 0.5 mM CaSO4 and immersed into basal solution. The composition of basal solution was based on being 0.1-strength for macronutrients and 0.25-strength for micronutrients of the nutrient solution used by McDonald et al. (Citation2001) to grow Triticeae species. Composition of the basal solution was (µM): CaCl2, 250; CaSO4.2 H2O, 250; MgSO4.7 H2O, 40; NH4NO3, 62.5; KH2PO4, 20; Fe-EDTA, 50; KNO3, 375; KCl, 12.5; H3BO3, 6.25; MnSO4.H2O, 0.5; ZnSO4.7 H2O, 0.5; CuSO4.5 H2O, 0.125; Na2MoO4.2 H2O, 0.125; NiSO4.7 H2O, 0.25; MES (buffer), 250. The pH of the solution was 6.5 at commencement of experiments and 6.6–6.9 at the end of experiments.
Leaf blades of FEFL were cut into 1–2 cm segments and immediately transferred to 125 mL Erlenmeyer flasks (sealed with cling wrap plastic) and incubated in 100 mL basal solution and PEG 8000 solution depending on the treatment. All flasks were put on a rotary shaker (100 opm) under continuous light (120 µmol quanta m−2 s−1, PAR) in a 20°C room.
2.2 Experiment 1: responses to potassium and sodium
Experiment 1 consisted of two PEG 8000 treatments (0 and −0.5 MPa), 4 nutrient solutions (basal solution, which contained 0.4 mM K+; basal plus 10 mM K+; basal plus 0.4 mM Na+; and basal plus 10 mM Na+), 2 sampling times (0 and 48 h) and 3 replicates, as maximum leaf OA was expressed under PEG −0.5 MPa at 48 h under these conditions. K+ and Na+ were both supplied with Cl−. The treatment of 0.4 mM K+ was within the high affinity range for uptake and 10 mM K+ was within the low affinity range (Epstein et al., Citation1963). High affinity for uptake enables absorption of ions from relatively low external concentrations. The affinity describes the capacity of the uptake mechanism to extract the ion from the medium and it is not directly a reflection of the amount of uptake. For Na+, the treatment of 0.4 mM Na+ was within high affinity for uptake mechanism and 10 mM Na+ was within the low-affinity range (Rains & Epstein, Citation1967).
2.3 Experiment 2: responses to nitrogen supply
Experiment 2 consisted of two PEG 8000 treatments (0 and −0.5 MPa), 4 nutrient solutions (basal solution, which contained 0.0625 mM NH4+ and 0.4 mM NO3−; basal solution without any N (i.e. sources of NH4+ and NO3− omitted); basal plus 0.4 mM NO3−; and basal plus 10 mM NO3−), 2 sampling times (0 and 48 h) and 3 replicates. Both treatments of 0.4 mM NO3− and 10 mM NO3− were supplied with K+. The treatments of 0.4 and 10 mM NO3− were selected as these are regarded as being in the high and low-affinity ranges for uptake (Crawford & Glass, Citation1998; Wang & Crawford, Citation1996).
2.4 Measurements
For measurements of leaf water content (WC) and relative water content (RWC), the leaves were rinsed for 2 min, using 0.5 mM CaSO4 and mannitol that had the same osmotic potential of expressed sap (OP) as the respective PEG treatment, so that leaf segments incubated in PEG −0.5 MPa were rinsed in mannitol −0.5 MPa. For measurements of leaf sap OP and solute concentrations, the leaves were rinsed for 2 min using 0.5 mM CaSO4 and mannitol that had the same OP as the respective PEG treatment, followed by 0.5 mM CaSO4 for 5 s. For OP measurement, the leaf tissues were placed in cryovials, frozen in liquid N2, and kept frozen until analysis. For measuring the concentration of inorganic and organic solutes, the leaf tissues were frozen in liquid N2 and freeze-dried, then extracted and analysed.
Leaf WC and leaf RWC were measured by weighing the fresh sample (excised leaf segments), floating the sample on 0.5 mM CaSO4 for 24 h in the dark at 20°C, blotting the sample, weighing turgid mass, drying in an oven for 48 h at 70°C, and re-weighing the dried sample. CaSO4 of 0.5 mM was used during the floating period to obtain turgid weight, as it can maintain membrane integrity and minimize solute leakage into the apoplast (Mengel & Kirby, Citation1979). Leaf WC and leaf RWC were calculated as:
WC (mL g−1) = (fresh mass – dry mass)/dry mass
RWC (%) = 100 × (fresh mass – dry mass)/(turgid mass – dry mass)
For measurements of leaf sap OP, leaf segments were put immediately into cryovials and sealed, and then vials were placed into liquid N2. Sap from samples (thawed while still in sealed vials) was squeezed using a simple press and 10 µL was analysed using a Fiske one-ten osmometer (Fiske Associates, Massachusetts USA) to determine OP using the method of freezing point depression.
Osmotic adjustment was calculated as the difference between the measured OP and the estimated OP as a result of any concentration-effect of any decreased tissue WC in PEG treatments. The concentration-effect on OP was the proportional decrease in leaf OP due only to the reduction in WC under PEG treatments and was equal to (WCcontrol/WCPEG) x OPcontrol. Despite RWC being used by others in these calculations, WC was used in the present study to calculate OA, because using RWC has a problem that the amount of water entering the apoplast/intercellular spaces of floating leaf tissues is uncertain (Boyer et al., Citation2008).
To calculate the contributions of individual solutes (measured as described below) to OA, the difference in concentration of individual solutes between PEG treatment and control was expressed on a molar basis in tissue water and then calculated as OP of extra solutes using the equation: OPextra solutes = M x R x T, where M is concentration in mol L−1, R is the universal gas constant and T is absolute temperature.
K+, Na+ and Cl−, were measured in freeze-dried leaf tissue samples. Dried samples were ground to a fine powder, and the ions were extracted from 0.1 g in 10 mL 0.5 M HNO3 (exact amounts of ground tissue and acid were recorded). After HNO3 was added to all samples, the samples in the vials were shaken in a 30°C room for 48 h. Diluted extracts were analysed for K+ and Na+ (Jenway PFP 7 flame photometer, Sherwood Scientific Ltd, Cambridge, UK) and for Cl− (Buchler-Cotlove chloridometer, Buchler Instruments Division Nuclear-Chicago Fort Lee, New Jersey, USA). Analyses were confirmed by taking a certified reference tissue through the same procedures.
Glycinebetaine, proline and total soluble sugars (TSS) were measured in the freeze-dried leaf tissues. Approximately 100 mg of lyophilized powder was accurately weighed into a 50 mL centrifuge tube. Three mL of ice-cold 5% (v/v) perchloric acid was added and mixed using a vortex mixer before being centrifuged at 15,000 rpm for 30 min (Fan et al., Citation1993). The supernatant was collected and stored in a sealed glass vial on ice. The pellet was extracted a second time in 3 mL of ice-cold 5% (v/v) perchloric acid, as before. The supernatants were combined and the pH was adjusted to between 3.0 and 3.5 using K2CO3 to precipitate the perchlorate. The sample was again centrifuged and the supernatant collected and the volume measured. The extract was filtered (0.22 μm) before injection into a HPLC (600 E pump and 717 plus autoinjector and 996 photodiode-array [PDA] detector, Waters Milford MA, USA) equipped with a Sugar-Pak column as described by Naidu (Citation1998), to measure glycinebetaine and proline. These compounds were quantified using standards. The same extract was used to measure TSS using anthrone reagent based on the method of Yemm and Willis (Citation1954).
2.5 Statistical analyses
Data were analysed using Genstat for Windows 8th Edition (Genstat software, VSN International, Hemel Hempstead, UK). Analysis of variance (ANOVA) was used to identify overall significant differences and interactions among treatments (where P < 0.05, unless otherwise stated). Standard errors were calculated using Microsoft Office Excel 2003.
3. Results
In Experiment 1, WC of the leaf segments increased with time in both zero and −0.5 MPa PEG treatments, but less water was absorbed at the lower OP (). There were no significant effects of treatments on RWC (). Leaf OP was more negative in the treatments of −0.5 MPa PEG and the addition of 10 mM KCl or NaCl resulted in the most negative OP (). Additional KCl and NaCl supply to the basal solution for incubating leaf segments of wheat in PEG at −0.5 MPa for 48 h affected OA expression, and leaf OA was about 100% higher with these additional ions than in basal solution with PEG −0.5 MPa (). The PEG treatments did not alter leaf [K+] in any of the KCl or NaCl treatments (), but Na+ (), Cl− (), glycinebetaine (), proline () and total soluble sugars () contributed to leaf OA in the presence of additional KCl and NaCl supply. Amongst these solutes, total soluble sugars were the biggest contributor (up to 40%) to OA, with Na+, Cl−, glycinebetaine and proline contributing up to 16, 15, 4 and 6%, respectively.
Figure 1. Water content (a), relative water content (b), expressed sap osmotic potential (c), and calculated osmotic adjustment (OA) (d) for segments of FEFL of wheat (cv. Hartog) in two PEG 8000 treatments (0 and − 0.5 MPa) in the basal incubation solution, basal plus 10 mM KCl, basal plus 0.4 mM NaCl, and basal plus 10 mM NaCl at 0 (initial) and 48 h of treatment. Values are means ± SE (n = 3). 0 MPa □, −0.5 MPa ■. Some error bars are too small to see. Significant differences (P < 0.05) between PEG treatments and amongst KCl and NaCl treatments were indicated by different letters.

Figure 2. Concentrations of K+ (a), Na+ (b), and Cl− (c) in segments of FEFL of wheat (cv. Hartog) in two PEG 8000 treatments (0 and − 0.5 MPa) in the basal incubation solution, basal plus 10 mM KCl, basal plus 0.4 mM NaCl and basal plus 10 mM NaCl at 0 (initial) and 48 h of treatment. Values are means ± SE (n = 3). 0 MPa □, −0.5 MPa ■. Some error bars are too small to see. Significant differences (P < 0.05) between PEG treatments and amongst KCl and NaCl treatments were indicated by different letters.

Figure 3. Concentrations of glycinebetaine (a), proline (b), and (c) total soluble sugars for segments of FEFL of wheat (cv. Hartog) in two PEG 8000 treatments (0 and − 0.5 MPa) in the basal incubation solution, basal plus 10 mM KCl, basal plus 0.4 mM NaCl, and basal plus 10 mM NaCl at 0 (initial) and 48 h of treatment. Values are means ± SE (n = 3). 0 MPa □, −0.5 MPa ■. Some error bars are too small to see. The scale of Y axis in Figure 3(c) is 3-fold that in Figure 3(a,b). Significant differences (P < 0.05) between PEG treatments and amongst KCl and NaCl treatments were indicated by different letters.

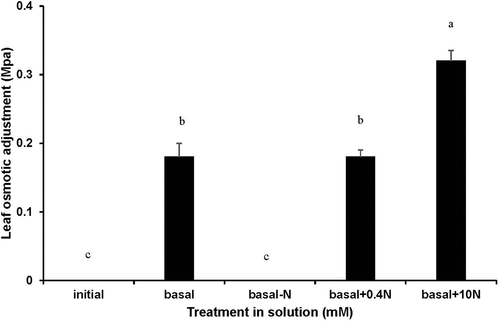
In Experiment 2, WC increased in the leaf segments over time in zero PEG treatments and decreased when the tissues were incubated on the −0.5 MPa PEG solutions (). RWC decreased over time in leaf segments as they floated on the −0.5 MPa PEG solution (). OP in leaf segments was more negative with time in −0.5 MPa PEG treatments (). There was no leaf OA expression in basal solution without N supply, whereas OA occurred in basal solution (0.18 MPa), basal plus 0.4 NO3− mM (0.18 MPa) and basal plus 10 mM NO3− (0.32 MPa) (). With N, leaf OA was more pronounced (about 80%) at basal plus 10 mM NO3− than at lower N supply.
Figure 4. Water content (a), relative water content (b), expressed sap osmotic potential (c), and calculated osmotic adjustment (OA) (d) for segments of FEFL of wheat (cv. Hartog) in two PEG 8000 treatments (0 and − 0.5 MPa) in the basal incubation solution, basal minus N, basal plus 0.4 mM NO3− and basal plus 10 mM NO3− at 0 (initial) and 48 h of treatment. Values are means ± SE (n = 3). 0 MPa □, −0.5 MPa ■. Some error bars are too small to see. Significant differences (P < 0.05) between PEG treatments and amongst N supply treatments were indicated by different letters.

[K+] in leaf segments decreased in −0.5 MPa PEG and adding 10 mM NO3− increased leaf [K+] (). [Na+] in the leaf segments increased as they floated in PEG solutions (). [Cl−] in the leaf segments decreased with time in both zero and −0.5 MPa PEG (). Initial leaf [Cl−] was 60% higher than in basal solution in zero PEG at 48 h (270 μmol g−1 DM). Extra leaf [Cl−] in basal, basal plus 0.4 mM NO3− and basal plus 10 mM NO3− accounted for 8, 20 and 4.4% of the respective OA. Leaf [Cl−] was about 30% of leaf [K+].
Figure 5. Concentrations of K+ (a), Na+ (b), and Cl− (c) in segments of FEFL of wheat (cv. Hartog) in two PEG 8000 treatments (0 and − 0.5 MPa) in the basal incubation solution, basal minus N, basal plus 0.4 mM NO3− and basal plus 10 mM NO3− at 0 (initial) and 48 h of treatment. Values are means ± SE (n = 3). 0 MPa □, −0.5 MPa ■. Some error bars are too small to see. The scale of Y axis in Figure 5(a) is 2-fold that in Figure 5(b,c). Significant differences (P < 0.05) between PEG treatments and amongst N supply treatments were indicated by different letters.

Leaf [glycinebetaine] decreased with time, especially in PEG treatments (). PEG treatment did not result in increased [glycinebetaine] in the leaf segments. Glycinebetaine did not contribute to OA. Leaf [proline] increased over time in PEG treatments (). Extra leaf [proline] in basal, basal plus 0.4 NO3− mM and basal plus 10 mM NO3− accounted for 1–7% of the OA. Leaf [proline] was about 50% of leaf [glycinebetaine]. Leaf [TSS] increased with time in PEG treatments (). Relative increases were largest in basal plus 10 mM NO3− and the lowest in basal solution. Extra leaf [TSS] in basal, basal plus 0.4 mM NO3− and basal plus 10 mM NO3− accounted for 18, 34 and 20% of OA, respectively. Leaf [TSS] (if mono-hexose) was about 6-fold higher than leaf [glycinebetaine].
Figure 6. Concentrations of glycinebetaine (a), proline (b), and total soluble sugars (c) for segments of FEFL of wheat (cv. Hartog) in two PEG 8000 treatments (0 and − 0.5 MPa) in the basal incubation solution, basal minus N, basal plus 0.4 mM NO3− and basal plus 10 mM NO3− at 0 (initial) and 48 h of treatment. Values are means ± SE (n = 3). 0 MPa □, −0.5 MPa ■. Some error bars are too small to see. The scale of Y axis in Figure 6(c) is 3-fold that in Figure 6(a,b). Significant differences (P < 0.05) between PEG treatments and amongst N supply treatments were indicated by different letters.

4. Discussion
4.1 OA and solutes in leaf segments in response to KCl and NaCl addition
In Experiment 1, the addition of 10 mM KCl or NaCl to basal solution containing PEG −0.5 MPa increased leaf OA by 100% over PEG −0.5 MPa alone (), with no change in leaf [K+] (), but with TSS, Na+, Cl−, glycinebetaine and proline increasing, and contributing 40, 16, 15, 4 and 6% to leaf OA ( respectively). Glass (Citation1983) suggested that when [K+] was low, high concentration of sugar and possibly unknown solutes reflected the capacity to substitute for the osmotic function normally performed by K+. However, higher K+ supply in the fertilizer of three tropical grasses grown in the field did not enhance leaf OA under water deficit (Wilson & Ludlow, Citation1983), because the difference of [K+] in the soil solution at field capacity between high (0.6 mM) and normal (0.4 mM) treatments was probably insufficient to affect OA expression. Here, addition of KCl and NaCl up to 10 mM in the incubation solution increased solute concentration and the development of OA in leaf segments of wheat ().
Additional KCl and NaCl supply to the basal solution influenced [K+] and [Na+] in leaf segments. Adding 10 mM KCl to the solution resulted in higher [K+] in leaf segments (), and this process showed low affinity of K+ uptake (Epstein et al., Citation1963). Addition of 10 mM NaCl decreased [K+] and increased [Na+] in leaf segments (). PEG −0.5 MPa treatments with additional KCl and NaCl supply to the basal solution increased [Cl−] in leaf segments (). Cl− is one of the inorganic solutes which are responsible for most of the tissue OP in several species under WD (Pugnaire et al., Citation1999), and [Cl−] increased under WD in fully-expanded wheat leaves in controlled environment (Munns et al., Citation1979). Cl− contributed 4 to 20% of OA in leaf segments.
4.2 OA and solutes in leaf segments in response to nitrogen omission and nitrate addition
The level of N supply in the solution for incubating leaf segments of wheat influenced the leaf OA expression in PEG −0.5 MPa after 48 h. Leaf OA was not found in the basal solution without macronutrient N, but it occurred in the other treatments, with 81% higher OA in the 10 mM NO3− treatment (0.32 MPa) than in basal solution in PEG −0.5 MPa. Na+, Cl−, proline and TSS contributed to leaf OA. As in Nio et al. (Citation2018), TSS were the largest contributor to OA (up to 34%), whereas proline was the smallest (up to 7%). Similarly in rice, higher N supply as NH4NO3 (4-times) in the incubation solution resulted in 50% higher leaf OA in 2-week-old intact plants under PEG 6000 with osmotic potential −0.06 lowered stepwise to −0.6 MPa (Yambao & O’Toole, Citation1984). The OA in leaves of rice were 0.26 MPa and 0.43 MPa in low N (0.7 mM) and high N (2.9 mM) supply, respectively; however, the solutes involved were not reported. The omission and addition of N in the incubation solution affected the OA expression in leaf segments of wheat subjected to PEG-induced WD.
Higher supply of NO3− did not enhance accumulation of proline or glycinebetaine in leaf segments of wheat incubated in PEG −0.5 MPa for 48 h. By contrast, leaf [proline] was up to 60% higher in barley seedlings grown in −1.2 MPa PEG treatment with 100% Knop solution (8 mM NO3−) than in 25% Knop solution (2 mM NO3−)(Kocheva et al., Citation2007). Information on leaf[glycinebetaine] accumulation in response to different N supply in PEG treatments is lacking. The accumulation of N-containing solutes, such as proline and glycinebetaine in this study, did not occur with higher [NO3−]supply either because(1)NO3− influx decreased and NO3− efflux increased (no NO3− uptake by leaf segments) with higher NO3− supply as shown in wheat seedlings (Jackson et al., Citation1976),or(2) NO3− uptake by leaf segments occurred, but it was not used for synthesis of proline and glycinebetaine.[NO3−]in leaf tissues still need to be tested, however, there was no tissue available after the other analyses, so NO3− levels could not be determined.
4.3 Solutes accumulated in leaf segments exposed to −0.5 MPa PEG
K+ was not accumulated in the leaf segments in −0.5 MPa PEG, whereas it was the major contributor to OA for intact leaves of wheat under WD (Morgan, Citation1984; Morgan, Citation1992; Munns et al., Citation1979; Nio et al., Citation2011). Therefore, K+ would be lost from the cells or there was no K+ uptake by leaf segments in PEG treatment ().
[Na+] was higher in PEG −0.5 MPa than in zero PEG (). The decrease in [Na+] in solution with −0.5 MPa PEG after 48 h of treatment indicated there was Na+ uptake by leaf tissues. Similarly, Na+ accumulated in Panicum maximum (green panic) subjected to a continuous 35 d drying cycle in the field (Ford & Wilson, Citation1981).
[Proline] in leaf segments in −0.5 MPa PEG was higher than in zero PEG (), but the proline accumulation was small. [Proline] increased up to 36-times higher (36 μmol g−1 DM) in leaves of barley seedlings treated with PEG 8000 (−0.8 MPa), but it accounted for less than 5% of OA after 24 h (Riazi et al., Citation1985). Although proline contribution to OA was minor, the observed increase in [proline] might have other positive roles, such as stabilization of enzyme structure and activity and protection of membrane integrity from damage by reactive oxygen species (Ashraf & Fooland, Citation2007; Chaves et al., Citation2003). In experiment 1, increased [proline] in leaf segments () was associated with less leakage of inorganic solutes from the leaf segments. Thus, proline accumulated in leaf segments of wheat under WD () and in intact plants in a controlled environment (Nio et al., Citation2011), but in all cases, contributed at most 7% of OA on a whole tissue basis, so these other roles of proline might be more important for tolerance of WD than its role in OA (Chaves et al., Citation2003; Riazi et al., Citation1985).
TSS accumulated in leaf segments of wheat subjected to −0.5 MPa PEG for 48 h. Increases in [TSS] could result from degradation processes (e.g. enhanced starch degradation), de novo synthesis (e.g. increased formation of hexose), or reduced consumption of photosynthate as growth is impeded (Arndt et al., Citation2001). Sugars accounted for OA in wheat grown at high radiance in the phytotron after 5 d of water deficit (Munns & Weir, Citation1981), with sucrose accounting for 70–90% of total sugars in expanded leaves. Munns and Weir (Citation1981) concluded that soluble sugars are important for acclimation during WD. Accumulation of sugars such as trehalose, sucrose, and raffinose can also help protect membrane integrity under cellular WD, by replacing water molecules (Bohnert et al., Citation1995).
This study examined OA and solutes accumulated in leaf segments of wheat exposed to PEG-induced WD in laboratory experiments. Maximum leaf OA (0.37 MPa) was expressed on PEG −0.5 MPa after 48 h, but not at −1.0 MPa or lower (Nio et al., Citation2018). In experiment 1, additional 10 mM K+ or Na+ added to basal solution with PEG −0.5 MPa enhanced leaf OA by up to 100%. K+ was not accumulated in leaf segments exposed to WD under −0.5 MPa PEG during 48 h, but Na+, Cl−, glycinebetaine, proline and TSS accounted for up to 16, 15, 4, 6 and 40% of OA, respectively. In experiment 2, the omission of nitrogen from the basal solution suppressed the expression of OA in leaf segments, but the addition of 10 mM NO3– increased leaf OA by up to 81%. K+ and glycinebetaine were not accumulated in leaf segments exposed to WD under PEG − 0.5 MPa during 48 h, but Na+, Cl−, proline and TSS accounted for up to 21, 20, 7 and 40% of OA, respectively. As proline only contributed 1–7% of OA on a whole-tissue basis, its other roles in protecting cell integrity may be more important. TSS were the largest contributor to OA and may also contribute to membrane stability. Further data are needed on accumulation of leaf NO3− and glycinebetaine in response to increasing nitrogen supply, and which other solutes may contribute to OA under PEG-induced WD. Laboratory experiments with leaf segments exposed to WD under −0.5 MPa PEG, and with 10 mM K+, Na+ or NO3− added to the treatment solution, could provide an effective and rapid pre-screen of diverse germplasm sources for OA expression. Selected genotypes should then be validated by exposure to soil WD for agronomic evaluation, and for OA expression under field WD.
Abbreviations
| DM | = | dry matter |
| FEFL | = | fully-expanded flag leaf |
| OA | = | osmotic adjustment |
| OP | = | osmotic potential |
| PEG | = | polyethylene glycol |
| RWC | = | relative water content |
| [Solute] | = | solute concentration |
| TSS | = | total soluble sugars |
| WC | = | water content |
| WD | = | water deficit. |
Acknowledgments
SAN received an ADS AusAID PhD scholarship. Special thanks to Dr. Tony Condon for seeds, Mr. Gunawan Wibisono, Ms. Rachel Javahar, Ms. Nadia Bazihizina and Ms. Wang Xing for help with sample collection, Mr. Greg Cawthray for HPLC analyses, Professors Hank Greenway and David Turner for critiquing the manuscript, and Professor Tim Colmer for supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
[dataset] Wade, Len (2022). Solute contributions to osmotic adjustment in leaf segments of wheat (Triticum aestivum L.) exposed to polyethylene glycol-induced water deficit. Mendeley Data, V1. http://dx.doi.org/10.17632/hm4d3pz6mb.1.
References
- Arndt, S. K., Clifford, C., Wanek, W., Jones, H. G., & Popp, M. (2001). Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiology, 21(11), 705–715. https://doi.org/10.1093/treephys/21.11.705
- Ashraf, M., & Fooland, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59(2), 206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
- Blum, A. (1988). Plant breeding for stress environments. CRC Press.
- Blum, A. (1998). Use of PEG to induce and control plant water deficit in experimental hydroponics’ culture. Retrieved October 8, 2022, from http://plantstress.com/use-of-peg/.
- Bohnert, H. J., Nelson, D. E., & Jensen, R. G. (1995). Adaptations to environmental stresses. The Plant Cell, 7(7), 1099–1111. https://doi.org/10.2307/3870060
- Boyer, J. S., James, R. A., Munns, R., & Condon, A. G. (2008). Osmotic adjustment leads to anomalously low estimates of relative water content in wheat and barley. Functional Plant Biology, 35(11), 1171–1182. https://doi.org/10.1071/FP08157
- Bressan, R. A., Hasegawa, P. M., & Handa, A. K. (1981). Resistance of cultured higher plant cells to polyethylene glycol-induced water stress. Plant Science Letters, 21(1), 23–30. https://doi.org/10.1016/0304-4211(81)90065-1
- Chaves, M. M., Maroco, J. P., & Pereira, J. S. (2003). Understanding plant responses to drought — from genes to the whole plant. Functional Plant Biology, 30(3), 239–264. https://doi.org/10.1071/FP02076
- Crawford, N. M., & Glass, A. D. M. (1998). Molecular and physiological aspects of nitrate uptake in plants. Trends in Plant Science, 3(10), 389–395. https://doi.org/10.1016/S1360-1385(98)01311-9
- Epstein, E., Rains, D. W., & Elzam, O. E. (1963). Resolution of dual mechanisms of potassium absorption by barley roots. Proceedings of the National Academy of Sciences 49, 684–692.
- Fan, T. W. M., Colmer, T. D., Lane, A. N., & Higashi, R. M. (1993). Determination of metabolites by 1H-NMR and GC: Analysis for organic osmolytes in crude tissue extracts. Analytical Biochemistry, 214(1), 260–271. https://doi.org/10.1006/abio.1993.1486
- Ford, C. W., & Wilson, J. R. (1981). Changes in levels of solutes during osmotic adjustment to water stress in leaves of four tropical pasture species. Australian Journal of Plant Physiology, 8(1), 77–91. https://doi.org/10.1071/PP9810077
- Glass, A. D. M. (1983). Regulation of ion transport. Annual Review of Plant Physiology, 34(1), 311–326. https://doi.org/10.1146/annurev.pp.34.060183.001523
- Hsu, S. Y., & Kao, C. H. (2003). The protective effect of free radical scavengers and metal chelators on polyethylene glycol-treated rice leaves. Biologia Plantarum, 46(4), 617–619. https://doi.org/10.1023/A:1024888217021
- Jackson, W. A., Kwik, K. D., Volk, R. J., & Butz, R. G. (1976). Nitrate influx and efflux by intact wheat seedlings: Effects on prior nitrate nutrition. Planta, 132(2), 149–156. https://doi.org/10.1007/BF00388896
- Jones, M. M., Turner, N. C., & Osmond, C. B. (1981). Mechanisms of drought resistance. In L. G. Paleg & D. Aspinall (Eds.), The physiology and biochemistry of drought resistance in plants (pp. 15–37). Academic Press.
- Kaufmann, M. R., & Eckard, A. N. (1971). Evaluation of water stress control with polyethylene glycol. Science, 133, 1486–1487.
- Kocheva, K. V., Georgiev, G., & Vunkova-Radeva, V. (2007). Contribution of mineral nutrition to the response of barley seedlings to polyethylene glycol–induced mild water stress. Journal of Plant Nutrition and Soil Science, 170(3), 392–397. https://doi.org/10.1002/jpln.200625182
- Lascano, H. R., Antonicelli, G. E., Luna, C. M., Melchiorre, M. N., Gómez, L. D., Racca, R. W., Trippi, V. S., & Casano, L. M. (2001). Antioxidant system response of different wheat cultivars under drought: Field and in vitro studies. Australian Journal of Plant Physiology, 28(11), 1095–1102. https://doi.org/10.1071/PP01061
- McDonald, M. P., Galwey, N. W., & Colmer, T. D. (2001). Waterlogging tolerance in the tribe triticeae: The adventitious roots of critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell and Environment, 24(6), 585–596. https://doi.org/10.1046/j.0016-8025.2001.00707.x
- Mengel, K., & Kirby, E. A. (1979). Principles of plant nutrition (2nd ed.). International Potash Institute.
- Morgan, J. M. (1984). Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology, 35(1), 299–319. https://doi.org/10.1146/annurev.pp.35.060184.001503
- Morgan, J. M. (1992). Osmotic components and properties associated with genotypic differences in osmoregulation in wheat. Australian Journal of Plant Physiology, 19(1), 67–76. https://doi.org/10.1071/PP9920067
- Munns, R., Brady, C. J., & Barlow, E. W. R. (1979). Solute accumulation in the apex and leaves of wheat during water stress. Australian Journal of Plant Physiology, 6(3), 379–389. https://doi.org/10.1071/PP9790379
- Munns, R., & Weir, R. (1981). Contribution of sugars to osmotic adjustment in elongating and expanded zones of wheat leaves during moderate water deficits at two light levels. Australian Journal of Plant Physiology, 8(1), 93–105. https://doi.org/10.1071/PP9810093
- Naidu, B. P. (1998). Separation of sugars, polyols, proline analogues, and betaines in stressed plant extracts by high performance liquid chromatography and quantification by ultra violet detection. Australian Journal of Plant Physiology, 25(7), 793–800. https://doi.org/10.1071/PP97165
- Naidu, B. P., Paleg, L. G., Aspinall, D., Jennings, A. C., & Jones, G. P. (1990). Rate of imposition of water stress alters the accumulation of nitrogen-containing solutes by wheat seedlings. Australian Journal of Plant Physiology, 17(6), 653–664. https://doi.org/10.1071/PP9900653
- Nio, S. A., Cawthray, G. R., Wade, L. J., & Colmer, T. D. (2011). Patterns of solutes accumulated during leaf osmotic adjustment as related to duration of water deficit for wheat at the reproductive stage. Plant Physiology and Biochemistry, 49(10), 1126–1137. https://doi.org/10.1016/j.plaphy.2011.05.011
- Nio, S. A., Ludong, D. P. M., & Wade, L. J. (2018). Comparison of leaf osmotic adjustment expression in wheat (Triticum aestivum L.) under water deficit between the whole plant and tissue levels. Agriculture and Natural Resources, 52(1), 33–38. https://doi.org/10.1016/j.anres.2018.03.003
- Pugnaire, F. I., Serrano, L., & Pardos, J. (1999). Constraints by water stress on plant growth. In M. Passarakli (Ed.), Handbook of plant and crop stress (Vol. 2, pp. 271–283). Marcel Decker.
- Rains, D. W., & Epstein, E. (1967). Sodium absorption by barley roots: Role of the dual mechanisms of alkali cation transport. Plant Physiology, 42(3), 314–318. https://doi.org/10.1104/pp.42.3.314
- Riazi, A., Matsuda, K., & Arslan, A. (1985). Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. Journal of Experimental Botany, 36(11), 1716–1725. https://doi.org/10.1093/jxb/36.11.1716
- Wang, R., & Crawford, N. M. (1996). Genetic identification of a gene involved in constitutive, high-affinity nitrate transport in higher plants. Proceedings of the National Academy of Sciences 93, 9297–9301.
- Wilson, J. R., & Ludlow, M. M. (1983). Time trends of solute accumulation and the influence of potassium fertilizer on osmotic adjustment of water-stressed leaves of three tropical grasses. Australian Journal of Plant Physiology, 10(6), 523–537. https://doi.org/10.1071/PP9830523
- Yambao, E. B., & O’Toole, J. C. (1984). Effects of nitrogen nutrition and root medium water potential on growth, nitrogen uptake and osmotic adjustment of rice. Physiologia Plantarum, 60(4), 507–515. https://doi.org/10.1111/j.1399-3054.1984.tb04919.x
- Yemm, E. W., & Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochemistry Journal, 57(3), 508–514. https://doi.org/10.1042/bj0570508
- Zhang, J., Nguyen, A., & Blum, H. T. (1999). Genetic analysis of osmotic adjustment in crops. Journal of Experimental Botany, 50(332), 291–302. https://doi.org/10.1093/jxb/50.332.291