ABSTRACT
Phosphorus (P) deficiency severely constrains rice production in sub-Saharan Africa. Previous studies showed that P-dipping, involving localized application of P near the root zone, and MP3, a natural allele of OsTB1/FC1 that enhances rice tillering, effectively improve rice growth and productivity in P-deficient soils in Madagascar. In the present study, we investigated the combined impact of these two technologies on the initial growth of transplanted rice in P-deficient soils using potted plants. Our experiments revealed that near-isogenic lines for MP3 and fc1 (the loss-of-function allele of OsTB1/FC1) promoted tillering and increased initial shoot biomass compared with the parental cultivar ‘Takanari’ when combined with P-dipping. The rise in shoot biomass would be attributed to increased P-uptake in the shoots, which were brought about by a continuous P-supply from the root zone due to P-dipping. Consequently, these combinations have the potential to enhance rice productivity in P-deficient paddy fields in sub-Saharan Africa.
Introduction
Phosphorus (P) deficiency is a significant obstacle to rice production in sub-Saharan Africa due to low soil P-content and the high P-fixing capacity of active Al- and Fe-oxides (Bekunda et al., Citation2010; Nishigaki et al., Citation2019; Saito et al., Citation2019). P-deficiency restricts rice tillering and stunts plant growth and development, which leads to low grain yield (Andrianary et al., Citation2021; Dobermann & Fairhurst, Citation2000). Although the application of fertilizers can enhance rice production, local farmers often lack the financial means to purchase sufficient amounts (Vanlauwe et al., Citation2014), resulting in inadequate fertilization practices (Tsujimoto et al., Citation2019). Moreover, P is a finite and nonrenewable resource and may be depleted within this century (Cordell et al., Citation2009). Therefore, it is crucial to develop effective P-fertilizer application practices (Rakotoson et al., Citation2022) and cultivate rice varieties adapted to P-deficient environments (Ismail et al., Citation2007).
Our recent studies in the central highlands of Madagascar have demonstrated that dipping rice seedlings in P-enriched slurry during transplanting promotes the initial growth of rice plants, resulting in an increase in grain yield in P-deficient lowlands (Rakotoarisoa et al., Citation2020). The P-dipping method creates a soluble P-hotspot around the transplanted roots, enabling efficient P-uptake even in high P-fixing soils and improving P-use efficiency (Oo, Tsujimoto, Rakotoarisoa, Kawamura, et al., Citation2020). Currently, efforts are under way to disseminate optimized P-dipping practices to local farmers in Madagascar (Oo & Tsujimoto, Citation2023).
Our previous studies have identified MP3 as a quantitative trait locus (QTL) and a natural allele of OsTB1/FC1 that moderately promotes rice tillering and increases panicle number (Takai et al., Citation2014, Citation2023). Given the limited tillering and panicle formation under P-deficiency, this QTL’s function may mitigate yield losses. On-farm trials conducted in the P-deficient lowlands of Madagascar confirmed that the near-isogenic line with MP3 (NIL-MP3) exhibited a 1.2-fold higher tiller/panicle number compared with its parent cultivar ‘Takanari’ (Takai et al., Citation2021). Furthermore, a NIL carrying fc1, the loss-of-function allele of OsTB1/FC1 (NIL-fc1), markedly enhanced tillering by 2.4-fold compared with ‘Takanari’ in nutrient-rich lowlands, despite lodging during grain filling (Takai et al., Citation2023). Considering the severe limitation of tillering in P-deficient environments, fc1 may be a more favorable choice to achieve higher tiller/panicle numbers.
This study aims to investigate the combined effect of P-dipping and tillering QTL on the initial growth of potted rice plants in P-deficient soils. By integrating these agronomic and breeding approaches, a more efficient rice cultivation system for P-deficient lowlands would be established.
Materials and methods
Plant materials
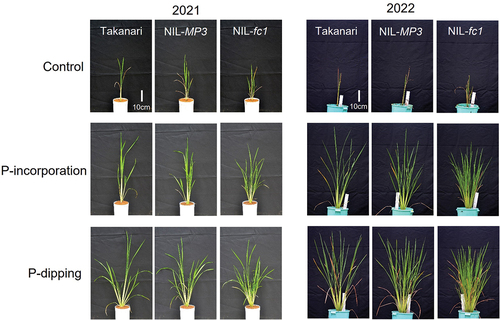
Two-year greenhouse pot experiments were conducted at the Japan International Research Center for Agricultural Science (JIRCAS) in Tsukuba, Japan (36°3′N, 140°5′E) from May to July in 2021 and 2022. The average daily temperature in the greenhouse was 25.4°C–33.2°C throughout the experimental periods. The study included three rice genotypes:”Takanari,” NIL-MP3, and NIL-fc1. ‘Takanari’ is a high-yielding indica cultivar in Japan (Imbe et al., Citation2004). NIL-MP3 (Takai et al., Citation2021) and NIL-fc1 (Takai et al., Citation2023) are near-isogenic lines with homozygosity for the Koshihikari allele and the loss-of-function allele of OsTB1/FC1, respectively, in the genetic background of ‘Takanari.’ OsTB1/FC1 is a gene that encodes a TCP family transcription factor that functions as a negative regulator of tiller bud outgrowth (Minakuchi et al., Citation2010; Takeda et al., Citation2003). After soaking seeds of the three rice genotypes in water at 30°C for two days, the germinated seeds were sown with one seed per cell in seedling cell trays filled with granular soils. Then, 15-day-old seedlings were transplanted with one seedling per pot on 10 May 2021, and 9 June 2022. Rice plants were cultivated under flooded conditions.
Soil conditions and P-treatments
Volcanic soil was collected from the forest subsoil (20–40 cm layer) in the experimental farm of JIRCAS to ensure a low availability of P. The soil was air-dried and sieved through an 8.0 mm mesh. It exhibited sandy loam characteristics with a pH of 5.7 and a high P-retention capacity of 99%, as reported by Oo, Tsujimoto, Rakotoarisoa, Kawamura, et al. (Citation2020) along with other soil properties. In the 2021 and 2022 experiments, 1/10000a and 1/5000a plastic pots were filled with 1 kg and 3 kg of prepared soil, respectively. Prior to transplanting, ammonium nitrate (NH4NO3) and potassium sulfate (K2SO4) were applied at rates of 100 mg nitrogen (N) kg−1 soil and 100 mg potassium (K) kg−1 soil, respectively, to ensure an adequate supply of N and K.
A randomized complete block design with five replications was used, combining three P-application methods, i.e. no P-application (control), P-incorporation, and P-dipping, with three rice genotypes. For the P-incorporation treatment, triple super phosphate [TSP; 42% phosphorus pentoxide (P2O5)] was added to the soil at a rate of 250 mg P2O5 kg−1 soil during puddling. For the P-dipping treatment, a soil slurry was prepared by mixing 10 g of TSP, 100 g of experimental soil, and 50–70 ml of distilled water following the method described by Oo, Tsujimoto, and Rakotoarisoa (Citation2020). Rice seedlings were then dipped in the slurry for a few minutes, and seedlings with slurry attached to their roots were transplanted into the pots. The estimated amount of P transferred through P-dipping at transplanting (P-enriched slurry attached to seedling roots) was approximately 90 mg P2O5 pot−1.
Measurements
The number of tillers was recorded, and plant samples were collected at 25 days (early tillering stage) and 39 days (late tillering stage) after transplanting in 2021 and 2022, respectively. To determine shoot biomass, the aboveground parts were cut at ground level and oven-dried at 70°C for two days. The shoot P-concentration was measured using the molybdate blue method (Murphy & Riley, Citation1962) after dry ashing at 550°C for 2 h and digesting with 0.5 M HCl. The shoot P-content (mg pot−1) was calculated by multiplying the P-concentration by the shoot biomass. After removing the shoots, the roots were thoroughly washed with tap water and preserved in 50% ethanol for subsequent measurements. The roots were scanned using an Epson Pro-selection X980 scanner, and the images were analyzed using WinRhizo Pro (Regent Instrument, Quebec, Canada) to estimate the total root length and root surface area. After root analysis, the root samples were oven-dried at 70°C for two days, and their biomass was determined.
Statistics
Statistical analyses were performed using a general linear model through SPSS 23.0 software (IBM, Chicago, USA). Two-way ANOVA was used to assess the effect of P-application, genotype, and their interaction on the measured variables for each year. P-application, genotype, and their interaction were considered fixed effects, whereas replication was considered a random effect. Significance of fixed effects was assessed using Tukey’s honestly significant difference test at a 5% level.
Results
Tiller development
ANOVA revealed significant and consistent effects of P-application, genotype, and their interaction on tiller number during the sampling periods in both 2021 and 2022 (). P-dipping resulted in the highest number of tillers, followed by P-incorporation, whereas the control had the lowest count. Among the genotypes, NIL-fc1 consistently exhibited the highest number of tillers across all P-application treatments. Notably, in 2022, NIL-fc1 displayed a remarkable 6.2-fold increase in tiller number compared with that of ‘Takanari’ under the P-dipping treatment. Conversely, NIL-MP3 showed a significantly higher tiller count than”Takanari” only when combined with P-dipping.
Figure 1. Effects of three different P-application treatments on the number of tillers (a, b), shoot biomass (c, d), and P-content in shoots (e, f) in 2021 and 2022 among”Takanari,” NIL-MP3, and NIL-fc1. *, **, and *** indicate significant effects and interactions between P-application method (P) and genotype (G) at the 5%, 1%, and 0.1% levels, respectively. Different letters represent significant differences among genotypes across P-application treatments at the 5% level based on Tukey’s honestly significant difference test. Error bars represent standard deviation.

Shoot biomass, shoot P-content, and root development
ANOVA revealed significant effects of P-application on shoot biomass in both 2021 and 2022 (). P-dipping resulted in the highest shoot biomass, followed by P-incorporation, whereas the control had the lowest shoot biomass. In 2021 and 2022, P-dipping led to 2.4 and 2.3 times greater shoot biomass, respectively, compared with P-incorporation, despite the smaller amounts of applied P (90 mg P2O5 vs. 250 mg P2O5 pot−1 and 90 mg P2O5 vs. 750 mg P2O5 pot−1, respectively). In 2022, a significant interaction between P-treatment and genotype was observed for shoot biomass. Under the P-dipping treatment, NIL-MP3 exhibited a 12% increase and NIL-fc1 showed a significant 24% increase in shoot biomass compared with ‘Takanari.’ However, no significant genotype differences were found in shoot biomass under the control and P-incorporation treatments. Corresponding with our findings on shoot biomass, shoot P-content tended to increase by 22% in NIL-MP3 and significantly increased by 37% in NIL-fc1 compared with ‘Takanari’ combined with the P-dipping treatment, whereas no genotype differences were observed in the other P-treatments (). P-application also significantly influenced root-related traits, with P-dipping resulting in the highest root biomass, root length, and root surface area, followed by P-incorporation, whereas the control had the lowest values (). However, no genotypic differences were observed for these traits.
Table 1. Two-way ANOVA for root-related traits at 25 and 39 days after transplantation in 2021 and 2022, respectively, in”Takanari,” NIL-MP3, and NIL-fc1 grown in the pots with the three P-application treatments.
Shoot biomass and P-content per tiller
Considering the genotypic differences observed in shoot biomass and shoot P-content under the P-dipping treatment in 2022, we further examined these traits per individual tiller. ANOVA revealed significant effects of P-application, genotype, and their interaction on shoot biomass per tiller and P-content per tiller (). P-dipping resulted in the highest biomass and P-content per tiller, followed by P-incorporation, whereas the control showed the lowest values. However, in the case of P-dipping, NIL-MP3 showed decreases of 32% and 26% in biomass and P-content per tiller, respectively, compared with ‘Takanari.’ Similarly, NIL-fc1 showed a substantial decrease of 80% and 78% in biomass and P-content per tiller, respectively, compared with ‘Takanari.’
Figure 2. Effects of three different P-application treatments on shoot biomass per tiller (a) and P-content per tiller (b) in 2022 among”Takanari,” NIL-MP3, and NIL-fc1. *** indicates significant effects and interactions between P-application method (P) and genotype (G) at the 0.1% level. Different letters represent significant differences among genotypes across P-application treatments at the 5% level based on Tukey’s honestly significant difference test. Error bars represent standard deviation.

Discussion
In this study, we demonstrated the efficacy of P-dipping as a superior P-treatment for promoting tillering with significantly lower amounts of applied P compared with conventional P-incorporation. This result aligns with previous studies that attribute the positive effect of P-dipping on initial growth to the abundant P-supply from soluble P-hotspots near the root system (Oo, Tsujimoto, Rakotoarisoa, Kawamura, et al., Citation2020 and Oo, Tsujimoto, Mukai, Nishigaki, et al., Citation2021). Furthermore, our findings highlight that the fc1 allele is most effective in promoting the highest number of tillers, and when combined with P-dipping, it synergistically enhances shoot biomass and P-uptake, with the following order observed in 2022: ‘Takanari’ < NIL-MP3 < NIL-fc1. However, it is worth noting that shoot biomass did not increase in the P-dipping treatment in 2021, despite the increase in tiller number. This discrepancy might be attributed to the sampling timing, with 25 days after transplanting in 2021 likely representing the stage of tillering promotion before substantial tiller growth. As plants continued to grow and tillers enlarged with adequate nutrient availability, the continuous P-supply in the P-dipping treatment supported vigorous tillering and subsequent tiller growth in NIL-fc1 and NIL-MP3, leading to the genotypic difference observed in shoot biomass and P-uptake in 2022.
Although NIL-fc1 exhibited the highest number of tillers, the tillers were the smallest and contained the lowest amount of P. Takai et al. (Citation2023) observed the presence of thin tillers and lodging in NIL-fc1 during grain filling under nutrient-rich conditions. Considering our focus on nutrient-poor environments, such as P-deficient soils in Madagascar, where the average rice yield is 2.7 t ha−1 (FAOSTAT, Citation2021), it is important to consider the potential lodging issues of NIL-fc1 even under P-deficient conditions. In such scenarios, the combination of NIL-MP3 with P-dipping may be a more favorable approach for increasing yields under P-deficiency, as NIL-MP3 maintained approximately 70% of the biomass per tiller in ‘Takanari.’ Further field-based studies are required to investigate this matter and evaluate the combination of genotypes and P-dipping until maturity.
In conclusion, the combination of P-dipping and fc1 or MP3 alleles effectively promoted tillering and initial plant growth, likely through enhanced P-uptake. These findings have the potential to contribute to an increase in rice productivity in P-deficient paddy fields in sub-Saharan Africa.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andrianary, B. H., Tsujimoto, Y., Rakotonindrina, H., Oo, A. Z., Rabenarivo, M., Ramifehiarivo, N., & Razakamanarivo, H. (2021). Phosphorus application affects lowland rice yields by changing phenological development and cold stress degrees in the central highlands of Madagascar. Field Crops Research, 271, 108256. https://doi.org/10.1016/j.fcr.2021.108256
- Bekunda, M., Sanginga, N., & Woomer, P. L. (2010). Chapter four - restoring soil fertility in sub-saharan africa. Advances in Agronomy, 54, 183–236. https://doi.org/10.1016/S0065-2113(10)08004-1
- Cordell, D., Drangert, J. O., & White, S. (2009). The story of phosphorus: Global food security and food for thought. Global Environmental Change, 19(2), 292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
- Dobermann, A., & Fairhurst, T. (2000). Rice: Nutrient disorders and nutrient management. PPI, PPIC, and Los Baños. IRRI.
- FAOSTAT. (2021). Food and agriculture organization of the United Nations, https://www.fao.org/faostat/en/#home.
- Imbe, T., Akama, Y., Nakane, A., Hata, T., Ise, K., Ando, I., Uchiyama, H., Nakagawa, N., Furutachi, H., Horisue, N., Noto, M., Fujita, Y., Kimura, K., Mori, K., Takayanagi, K., Uehara, Y., Ishizaka, S., Nakagaura, M., Yamada, T., & Koga, Y. (2004). Development of a multipurpose high-yielding rice variety “takanari. Bulletin of the National Institute of Crop Science. 5, 35–51. in Japanese with English summary. https://www.naro.go.jp/publicity_report/publication/archive/files/5-3.pdf
- Ismail, A. M., Heuer, S., Thomson, M. J., & Wissuwa, M. (2007). Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Molecular Biology, 65(4), 547–570. https://doi.org/10.1007/s11103-007-9215-2
- Minakuchi, K., Kameoka, H., Yasuno, N., Umehara, M., Luo, L., Kobayashi, K., Hanada, A., Ueno, K., Asami, T., Yamaguchi, S., & Kyozuka, J. (2010). FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiology, 51(7), 1127–1135. https://doi.org/10.1093/pcp/pcq083
- Murphy, J., & Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
- Nishigaki, T., Tsujimoto, Y., Rinasoa, S., Rakotoson, T., Andriamananjara, A., & Razafimbelo, T. (2019). Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant and Soil, 435(1–2), 27–38. https://doi.org/10.1007/s11104-018-3869-1
- Oo, A. Z., & Tsujimoto, Y. (2023). Localized phosphorus application via P-dipping is more effective for improving initial rice growth in lower temperature conditions. Plant Production Science, 26(1), 28–35. https://doi.org/10.1080/1343943X.2022.2160363
- Oo, A. Z., Tsujimoto, Y., Mukai, M., Nishigaki, T., Takai, T., & Uga, Y. (2021). Synergy between a shallow root system with a DRO1 homologue and localized P application improves P uptake of lowland rice. Scientific Reports, 11(1), 9484. https://doi.org/10.1038/s41598-021-89129-z
- Oo, A. Z., Tsujimoto, Y., & Rakotoarisoa, N. M. (2020). Optimizing the phosphorus concentration and duration of seedling dipping in soil slurry for accelerating the initial growth of transplanted rice. Agronomy, 10(2), 240. https://doi.org/10.3390/agronomy10020240
- Oo, A. Z., Tsujimoto, Y., Rakotoarisoa, N. M., Kawamura, K., & Nishigaki, T. (2020). P-dipping of rice seedlings increases applied P use efficiency in high P-fixing soils. Scientific Reports, 10(1), 11919. https://doi.org/10.1038/s41598-020-68977-1
- Rakotoarisoa, N. M., Tsujimoto, Y., & Oo, A. Z. (2020). Dipping rice seedlings in P-enriched slurry increases grain yield and shortens days to heading on P-deficient lowlands in the central highlands of Madagascar. Field Crops Research, 254, 107806. https://doi.org/10.1016/j.fcr.2020.107806
- Rakotoson, T., Tsujimoto, Y., & Nishigaki, T. (2022). Phosphorus management strategies to increase lowland rice yields in sub-saharan Africa: A review. Field Crops Research, 275, 108370. https://doi.org/10.1016/j.fcr.2021.108370
- Saito, K., Vandamme, E., Johnson, J., Tanaka, A., Senthilkumar, K., Dieng, I., Akakpo, C., Gbaguidi, F., Segda, Z., Bassoro, I., Lamare, D., Gbakatchetche, H., Abera, B. B., Jaiteh, F., Bam, R. K., Dogbe, W., Sékou, K., Rabeson, R. … Wopereis, M. C. S. (2019). Yield-limiting macronutrients for rice in sub-Saharan Africa. Geoderma, 338, 546–554. https://doi.org/10.1016/j.geoderma.2018.11.036
- Takai, T., Ikka, T., Kondo, K., Nonoue, Y., Ono, N., Arai-Sanoh, Y., Yoshinaga, S., Nakano, H., Yano, M., Kondo, M., & Yamamoto, T. (2014). Genetic mechanisms underlying yield potential in the rice high-yielding cultivar Takanari, based on reciprocal chromosome segment substitution lines. BMC Plant Biology, 14(1), 295. https://doi.org/10.1186/s12870-014-0295-2
- Takai, T., Sakata, M., Rakotoarisoa, N. M., Razafinarivo, N. T., Nishigaki, T., Asai, H., Ishizaki, T., & Tsujimoto, Y. (2021). Effects of quantitative trait locus MP3 on the number of panicles and rice productivity in nutrient-poor soils of Madagascar. Crop Science, 61(1), 519–528. https://doi.org/10.1002/csc2.20344
- Takai, T., Taniguchi, Y., Takahashi, M., Nagasaki, N., Yamamoto, E., Hirose, S., Hara, N., Akashi, H., Ito, J., Arai-Sanoh, Y., Hori, K., Fukuoka, S., Sakai, H., Tokida, T., Usui, Y., Nakamura, H., Kawamura, K., Asai, H. … Uga, Y. (2023). MORE PANICLES 3, a natural allele of OsTB1/FC1, impacts rice yield in paddy fields at elevated CO2 levels. The Plant Journal: For Cell and Molecular Biology, 114(4), 729–742. https://doi.org/10.1111/tpj.16143
- Takeda, T., Suwa, Y., Suzuki, M., Kitano, H., Ueguchi-Tanaka, M., Ashikari, M., Matsuoka, M., & Ueguchi, C. (2003). The OsTB1 gene negatively regulates lateral branching in rice. The Plant Journal: For Cell and Molecular Biology, 33(3), 513–520. https://doi.org/10.1046/j.1365-313X.2003.01648.x
- Tsujimoto, Y., Rakotoson, T., Tanaka, A., & Saito, K. (2019). Challenges and opportunities for improving N use efficiency for rice production in sub-saharan africa. Plant Production Science, 22(4), 413–427. https://doi.org/10.1080/1343943X.2019.1617638
- Vanlauwe, B., Wendt, J., Giller, K. E., Corbeels, M., Gerard, B., & Nolte, C. (2014). A fourth principle is required to define conservation agriculture in sub-saharan Africa: The appropriate use of fertilizer to enhance crop productivity. Field Crops Research, 155, 10–13. https://doi.org/10.1016/j.fcr.2013.10.002