Abstract
Different plant extracts have been screened by thin-layer chromatography (TLC) bioautography in an effort to discover new acetylcholinesterase (AChE) inhibitors. The CH2Cl2 extract of Peucedanum ostruthium. (L.) Koch roots exhibited significant inhibition of AChE -l activity. Active constituents were isolated by bioguided fractionation using almost exclusively centrifugal partition chromatography. Four coumarins (ostruthin, imperatorin, ostruthol, and oxypeucedanin hydrate) and a chromone derivative (peucenin) were found to inhibit AChE activity in this bioassay.
Introduction
Alzheimer's Disease affects 18 million people worldwide (Alzheimer's Disease International, Citation2004) and is characterized by a progressive degeneration of cognitive functions, including loss of memory or inaptitude to carry out daily life tasks. One approach for treating this disease is to enhance the acetylcholine level in the brain using acetylcholinesterase (AChE; EC 3.1.1.7) inhibitors (Scarpini et al., Citation2003). Although the use of AChE inhibitors is one of the most efficient approaches to treat Alzheimer's Disease symptoms, current inhibitors, such as the synthetic drug donepezil or galanthamine from snowdrop (Galanthus nivalis. L., Amaryllidaceae), still present some disadvantages, such as a short half-life or side effects (Grossberg, Citation2003).
Until now, natural inhibitors of AChE have mainly been alkaloids (Zanagara, Citation2003; Elgorashi et al., Citation2004). In the hope that other classes of compounds should (RH induce fewer-adverse effects, a screening program was undertaken in an effort to discover new, non-alkaloid inhibitors of AChE, using a thin-layer chromatography (TLC) bioautographic assay (Marston et al., Citation2002). In this method, the plant constituents are first separated by TLC. Then, AChE is sprayed on the plate. After incubation at 37°C in order to reactivate the enzyme, a mixture of 1-naphthyl acetate and Fast Blue B salt is sprayed on the TLC plate. Active enzyme cleaves 1-naphthyl acetate to yield 1-naphthol, which in turn reacts with Fast Blue B salt to give a purple-colored diazonium dye. AChE inhibitors on the plate prevent the enzyme from reacting with 1-naphthyl acetate and stop formation of the purple coloration. Thus, areas corresponding to inhibitory compounds appear as white spots against a purple background.
During a plant screening program, a CH2Cl2 extract of roots from Peucedanum ostruthium. (L.) Koch (Apiaceae) was shown to contain several compounds inhibiting AChE activity. P. ostruthium. (L.) Koch (syn. Imperatoria ostruthium. L.) is a perennial herb found in Central Europe. The roots have been used in folk medicine for their stimulant, antispasmodic, analgesic, and carminative properties and also as a flavoring agent, the roots being spicier than pepper. Among all the properties accorded to P. ostruthium. roots, antimycobacterial, antiphlogistic, and antipyretic activities were shown to be due to coumarins (Hiermann & Schantl, Citation1998; Schinkovitz et al., Citation2003). Along with these compounds, P. ostruthium. also contains furanocoumarins, chromones, hesperidin, and phthalides (Hörhammer et al., Citation1969; Khaled et al., Citation1975; Gijbels et al., Citation1984).
Materials and Methods
General experimental procedures
Centrifugal partition chromatography (CPC) was performed on a CCC-1000 instrument (Pharma-Tech Research Corp., Baltimore, MD, USA). The total volume of the three coils was 650 ml, and the rotation speed was 1000 rpm. Elution was monitored at 254 nm with as Knauer Milford, MA, USA UV-Visible detector and a Tarkan (W & W, Basle, Switzerland) model 600 integrator. The flow rate was 3 ml/min. Fractions were collected with a LKB (Bromma, Sweden) 2070 Ultrorac II fraction collector. High performance liquid chromatography (HPLC)-UV was performed on a Hewlett-Packard (Palo Alto, CA, USA) 1100 instrument epuipped with a photodiode array detector and a Nova-Pak RP-18 column (4 µm, 150 × 3.9 mm i.d., Waters). A MeOH-H2O gradient (25:75 → 100:0) containing 0.05% trifluoroacetic acid (TFA) was applied at a flow rate of 1.0 ml/min during 25 min, followed by 5 min with 100% MeOH.
1H and 13C NMR spectra were run on a Varian (Palo Alto, CA, USA) Unity Inova 500 MHz NMR instrument; compounds were dissolved in acetone-d.6, except oxypeucedanin hydrate, which was dissolved in CD3OD; TMS was used as internal standard.
For liquid chromatography/mass spectrometry (LC/MS), the crude extract was analyzed under the same HPLC conditions as for analytical LC/UV. A Finnigan MAT (Bremen, Germany) LCQ detector was used, equipped with an atmospheric pressure chemical ionization (APCI) interface as the ionization source, which was operated under the following conditions: sheath gas, N2 with flow of 77 l/min; capillary temperature, 150°C; APCI vaporizer temperature, 450°C source voltage, 6 kV; source current, 5 µA; capillary voltage, 16 V; tube lens offset, 10 V; multipole 1 offset, −3.75 V; multipole 2 offset, −7.00 V; inter-multipole lens voltage, −10 V; trap DC offset voltage, −18 V. Full-scan mass spectra (150–500 amu) were recorded every 2 s in positive ion mode. The protonated molecule ions [M + H]+ of coumarins were chosen as the parent ions for isolation and fragmentation. MSn experimental conditions were as follows: He CID pressure, 0.5 mTorr; relative collision energy, 40% fragmentation time, 200 ms. The spectra were acquired every second, and the window was reduced to 50–500 amu.
Plant material
Roots of Peucedanum ostruthium. (L.) Koch were collected in Champex, Valais, Switzerland, in June 2001. A voucher specimen (2001034) is deposited at the Laboratory of Pharmacognosy and Phytochemistry, University of Geneva.
Extraction and isolation
Powdered roots (200 g) of P. ostruthium. were extracted successively with hexane (3 × 1.2 l, 24 h), and CH2Cl2 (3 × 1.2 l, 24 h) at room temperature, to give 6.9 and 8.6 g of extract, respectively.
A part of the CH2Cl2 extract (3.0 g) was dissolved in 40 ml of the biphasic system n.-hexane-EtOAc-MeOH-H2O (10:5:5:1) and then fractionated by CPC using the same solvent system. The upper phase was first used as mobile phase, giving seven fractions (I–VII). Four further fractions (VIII–XI) were subsequently obtained by elution in the reversed-phase mode (lower phase as mobile phase).
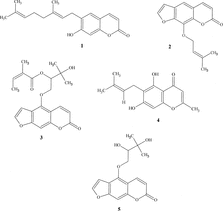
A second CPC on fraction III with the same solvent system gave three subfractions (III-1 to III-3). The fraction III-2 (370 mg), containing the main active compound, was then submitted to another CPC separation using n.-hexane-MeCN-CH2Cl2 (10:7:3; upper phase as mobile phase), leading to the isolation of 1 (22 mg) (). The purity of the compound was checked by TLC (Rf: 0.64) and HPLC (Rt: 22.5 min).
Another CPC on fraction IV with the ternary solvent system n.-hexane-t.-butyl methyl ether-MeCN (5:2:4, upper phase as mobile phase) led to the isolation of 2 (56 mg). The purity of the compound was checked by TLC (Rf: 0.52) and HPLC (Rt: 20.3 min).
Crystallization from EtOAc/hexane of fraction VII afforded 3 (67 mg). The purity of the compound was checked by TLC (Rf: 0.34) and HPLC Rt: 18.0 min).
Compound 4 (4 mg) was obtained from fraction VI after crystallization from EtOAc/hexane. The purity of the compound was checked by TLC (Rf: 0.43) and HPLC (Rt: 18.2 min).
Compound 5 (6 mg) was obtained by crystallization of fraction IX. The purity of the compound was checked by TLC (Rf: 0.11) and HPLC (Rt: 11.6 min).
Anti-acetylcholinesterase bioautographic assay
The TLC bioautographic method in this study was the same as described previously (Marston et al., Citation2002). Coumarins were dissolved in EtOAc. The solutions were applied to TLC in varying dilutions, and plates were developed with a mixture of hexane-EtOAc (1:1). Three known inhibitors of acetylcholinesterase were used as references compounds; huperzine A (product no. H 5777) and galanthamine HBr (product no. G 1660) were purchased from Sigma (St. Louis, MO, USA), and physostigmine (eserine) was purchased from Fluka (Buchs, Switzerland; product no. 45710).
Results
The crude CH2Cl2 extract of P. ostruthium. exhibited on TLC several very clear inhibition spots. The corresponding compounds were UV-active at 366 nm, indicating they were mainly coumarin derivatives.
Fractionation of the crude CH2Cl2 extract by centrifugal partition chromatography (CPC) afforded 11 fractions (I–XI) that were monitored by the TLC bioautographic method: fractions III to XI exhibited an AChE inhibitory activity, due to one or more compounds. Another CPC separation of fraction III gave three fractions (III-1 to III-3), and a second CPC step of fraction III-2 followed by crystallization from EtOAc/hexane afforded 1 (22 mg). CPC of fraction IV followed by crystallization from EtOAc/hexane also allowed the purification of compound 3 (67 mg) from fraction VII and compound 4 (4 mg) from fraction VI. Compound 4 was visible under UV light at 254 nm on TLC but was not detected at 366 nm. The UV spectrum of the isolated substance confirmed that this compound was not a coumarin derivative. Compound 5 (6 mg) was obtained by crystallization from fraction IX from EtOAc/hexane.
NMR, UV, and MS data of compounds 1–5 () matched those of ostruthin (1), imperatorin (2), Z.-ostruthol (3), peucenin (4), and oxypeucedanin hydrate (5). These products have previously been reported in P. ostruthium. (Khaled et al., Citation1975).
The pure compounds were then tested against AChE using the bioautographic assay. Solutions of pure compounds were applied on the TLC plate in a quantity of 1 µg. All the molecules gave an inhibition spot after spraying with enzyme and substrate. Dilutions were then made in order to find the minimum quantity required to produce white inhibition spots ().
Table 1.. Anti-acetylcholinesterase activity of coumarins from P. ostruthium. and reference inhibitors.
Discussion
The five isolated molecules (1–5) inhibited AChE in this TLC bioautographic assay. It was shown that the coumarins were more active than the chromone derivative peucenin. Moreover, in this bioassay, ostruthol, the most active of the four tested coumarins, was about 10-fold more active than the commercial AChE inhibitor galanthamine and as strong as huperzine A [from Huperzia serrata. (Thunb. ex Murray) Trevis, Lycopodiaceae], which is currently one of the most powerful natural AChE inhibitors.
Other coumarins from the Apiaceae (Angelica gigas. Nakai) were previously shown to inhibit AChE activity (Kang et al., Citation2001). Another class of non-alkaloidal compounds, the xanthones, has also given interesting results concerning inhibition of AChE activity (Urbain et al., Citation2004). By coupling CPC fractionation with bioautographic assay, four coumarins and a chromone derivative with acetylcholinesterase inhibitory activity have bneen obtained from Peucedanum ostruthium.. The diversity of the structures of active compounds makes it impossible at the moment to explain the relation between the structure and the inhibitory activity. The strong activity observed for ostruthol should be followed by further in vitro., in vivo., and toxicological testing, as this compound may have potential for the treatment of Alzheimer's Disease.
Acknowledgments
The Swiss National Science Foundation (grade no. 2153-066874.01/1 to K. Hostettmann) is gratefully acknowledged for financial support.
References
- Alzheimer's Disease International (2004): Annual Report 2003/2004, Alzheimer's Disease International, London.
- Elgorashi EE, Stafford GI, van Staden J (2004): Acetycholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. Planta Med 79: 258–260. [CSA]
- Gijbels MJM, Fischer FC, Scheffer JJC, Baerheim Svendsen A (1984): Phthalides in roots of Capnophyllum peregrinum. and Peucedanum ostruthium.. Planta Med 150: 110. [CSA]
- Grossberg GT (2003): Cholinesterase inhibitors for the treatment of Alzheimer's disease: Getting on and staying on. Curr Ther Res 64: 216–235. [CSA], [CROSSREF]
- Hiermann A, Schantl D (1998): Antiphlogistic and antipyretic activity of Peucedanum ostruthium.. Planta Med 64: 400–403. [PUBMED], [INFOTRIEVE], [CSA]
- Hörhammer L, Wagner H, Heydweiller D (1969): Hesperidin aus dem Rhizom von Peucedanum ostruthium.. Phytochemistry 8: 1605–1606. [CSA], [CROSSREF]
- Kang SY, Lee KY, Sung SH, Park MJ, Kim YC (2001): Coumarins isolated from Angelica gigas. inhibit acetylcholinesterase: Structure-activity relationships. J. Nat Prod 64: 683–685. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Khaled SA, Szendrei K, Novák I, Reisch J (1975): Coumarin glycosides from Peucedanum ostruthium.. Phytochemistry 14: 1461–1462. [CSA], [CROSSREF]
- Marston A, Kissling J, Hostettmann K (2002): A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem Anal 13: 51–54. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Scarpini E, Scheltens P, Feldman H (2003): Treatment of Alzheimer's disease: Current status and new perspectives. Lancet Neurol 2: 539–547. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schinkovitz A, Gibbons S, Stavri M, Cocksedge MJ, Bucar F (2003): Ostruthin: An antimycobacterial coumarin from the roots of Peucedanum ostruthium.. Planta Med 69: 369–371. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Urbain A, Marston A, Queiroz EF, Ndjoko K, Hostettmann K (2004): Xanthones from Gentiana campestris. as new acetylcholinesterase inhibitors. Planta Med 70: 1101–1014. [CSA], [CROSSREF]
- Zangara A (2003): The psychopharmacology of huperzine A: An alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease. Pharmacol Biochem Behav 75: 675–686. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]