Abstract
A total of 25 phenolic compounds were studied for their inhibitory effects against herpes simplex virus (HSV)-1, HSV-2, and human immunodeficiency virus (HIV)-1. These include five flavonoids (1–5) and two dimeric stilbenes (6,7) from Artocarpus gomezianus. Wall. ex tréc., five phloroglucinol derivatives (8–12) from Mallotus pallidus. (Airy Shaw) Airy Shaw, and 13 courmarins (13–25) from Triphasia trifolia. (Burm.f.) P. Wilson. The data obtained in this study suggest the bis-hydroxyphenyl structure as a potential target for anti-HSV and HIV drugs development.
Introduction
Herpes simplex virus (HSV) is a DNA virus responsible for a wide range of diseases, ranging from gingivostomatitis to keratoconjunctivitis, genital disease, encephalitis, and infection of newborn and immunocompromised patients (Jensen et al., Citation1997). Human immunodeficiency virus (HIV) is a retrovirus that so far has affected millions of people worldwide (Derdeyn & Silvestri, Citation2005). A number of natural phenolics have been shown to possess antiviral activities (Fukuchi et al., Citation1989; Lin et al., Citation1999; Wu et al., Citation2003; Likhitwitayawuid et al., Citation2005). In this communication, we report our studies on the anti-HSV and anti-HIV activities of phenolics obtained from Artocarpus gomezianus. Wall. ex tréc. (Moraceae), Mallotus pallidus. (Airy Shaw) Airy Shaw (Euphorbiaceae), and Triphasia trifolia.(Burm.f.) P. Wilson (Rutaceae).
Materials and Methods
General
Melting points were measured on a Fisher-Johns melting point apparatus (Pittsburgh, PA). IR spectra were recorded as KBr disks on a JASCO FT/IR-300E spectrophotometer (Tokyo, Japan), and UV spectra were measured on a Thermospectronic UV-1 spectrophotometer (New York, USA). 1H (500 MHz) and 13C (125 MHz) NMR spectra were recorded on a Bruker DPX 500 spectrometer (Rheinstetten, Germany). EI mass spectra were obtained with a Fison Micromass VG Platform II mass spectrometer (Altrincham, UK).
Extraction and isolation
A. gomezianus
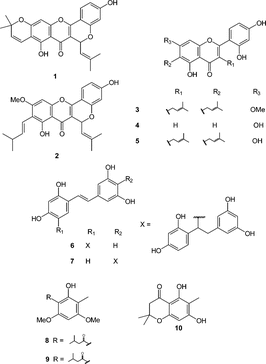
The isolation and identification of isocyclomorusin (1), cycloartocarpin (2), artocarpin (3), norartocarpetin (4), cudraflavone C (5), artogomezianol (6) and andalasin A (7) () have been earlier reported (Likhitwitayawuid et al., Citation2000; Likhitwitayawuid & Sritularak, Citation2001).
M. pallidus
The isolation and identification of pallidusol (8), dehydropallidusol (9), pallidol (10), mallopallidol (11), and homomallopallidol (12) ( and ) have been previously described (Supudompol et al., Citation2004; Likhitiwitaywuid & Supudompol, Citation2005).
T. trifolia
The leaves were collected from the Medicinal Plants Garden, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand, in April 2001. A voucher specimen has been deposited at the Herbarium of the Faculty of Pharmaceutical Sciences, Chulalongkorn University. Dried and ground leaves (1 kg) were extracted with hexane. The hexane extract (17 g) was fractionated on SiO2 with EtOAc-hexane gradient to give nine fractions (I–IX). Fraction II was further separated by column chromatography (SiO2, hexane-EtOAc gradient) and then purified by recrystallization from EtOAc to give aurapten (13; 15 mg). Fraction IV was subject to gel filtration chromatography (Sephadex LH-20, CHCl3-MeOH 1:1) to give four fractions (IVa–IVe). Separation of fraction IVb on Sephadex LH-20 (MeOH-CHCl3 1:1) and then on SiO2 (hexane-EtOAc 7:3) gave anisocoumarin E (16; 6 mg). Fraction IVc was separated on a Sephadex LH-20 (MeOH-CHCl3 1:1) column to give phellopterin (24; 6 mg). Gel filtration chromatography of fraction IVd (Sephadex LH-20, MeOH-CHCl3 1:1) gave bergapten (22; 3 mg). Fraction VI was fractionated on SiO2 (hexane-EtOAc gradient) to give nine fractions (VIa–VIi). Fraction VIb was separated on Sephadex LH-20 (MeOH) to give isopimpinellin (23; 5 mg), whereas fraction VIc was separated on Sephadex LH-20 (CHCl3-MeOH 1:1) to give isomeranzin (14; 60 mg). Separation of fraction VIe on Sephadex LH-20 (CHCl3-MeOH 1:1) gave isosibiricin (15; 53 mg). Column chromatography of fraction VIf (Sephadex LH-20, CHCl3-MeOH 1:1) gave isoponcimarin (17; 9 mg). Fraction VIg gave omphamurin (21; 12 mg) after separation on Sephadex LH-20 (MeOH). Fraction VII was fractionated on Sephadex LH-20 (MeOH) to give three fractions (VIIa–VIIc). Fraction VIIa was purified on Sephadex LH-20 (CHCl3-MeOH) to furnish triphasiol (18; 30 mg), while fraction VIIb was further separated on Sephadex LH-20 (MeOH) to give byakangelicin (25; 3 mg). Fraction VIII was subjected to column chromatography (SiO2, hexane-EtOAc) to give five fractions VIIIa–VIIIe). Fraction VIIId was separated on Sephadex LH-20 (CHCl3-MeOH 1:1) to give mexoticin (19; 3 mg) and meranzin hydrate (20; 7 mg). Identification of these isolates was carried out by comparison of their NMR and MS data with reported values for aurapten (13) (Chen et al., Citation1995), isomeranzin (14) (Abaul et al., Citation1994), isosibiricin (15) (Imai et al., Citation1989; Chen et al., Citation1995; Kinoshita et al., Citation1996), anisocoumarin E (16) (Ngadjui et al., Citation1989), isoponcimarin (17) (Guiotto et al., Citation1976), triphasiol (18) (Abaul et al., Citation1994; Ruangrungsi et al., Citation1994), mexoticin (19) (Ruangrungsi et al., Citation1994), meranzin hydrate (20) (Ruangrungsi et al., Citation1994), omphamurin (21) (Wu, Citation1981; Kinoshita et al., Citation1996), bergapten (22) (Bergendorff et al., Citation1997), isopimpinellin (23) (Basnet et al., Citation1993), phelloterin (24) (Bergendorff et al., Citation1997) and byakangelicin (25) (Fujioka et al., Citation1999).
Anti-HSV activity evaluation
Assays of anti-HSV activity
Antiviral activity against HSV-1 (Strain KOS) and HSV-2 (Strain 186) was determined using the plaque reduction method, as previously described (Likitwitayawuid et al., Citation2005). Briefly, virus (30 PFU/25 µl) was mixed with 25 µl of complete medium containing various concentrations of test compound and then incubated at 37°C for 1 h. After incubation, the mixtures were added to Vero cells (6 × 105 cells/well) in 96-well microtiter plates and incubated at 37°C for 2 h. The overlay medium containing the various concentrations of test compound was added to the Vero cells and incubated at 37°C in humidified CO2 incubator for 2 days. Then, virus growth inhibition was evaluated by counting the virus plaque forming on Vero cells compared with the controls. The cells also were stained with 1% crystal violet in 10% formalin for 1 h. The percent plaque inhibition was determined. Acyclovir was used as positive control.
Assays of anti-HIV activity
Anti-HIV assays were performed in human peripheral blood mononuclear cells (PBMCs) as earlier reported (Schinazi et al., Citation1990). Briefly, uninfected phytohemagglutinin-stimulated human PBMCs were infected with HIV-1 (strain LAV-1) (about 63,000 disintegrations of reverse transcriptase (RT) activity per minute per 107 cells per 10 ml of medium). The test samples were then added to duplicate or triplicate cultures. Uninfected and untreated PBMCs were grown in parallel at equivalent cell concentrations as controls. The cultures were maintained in humidified 5% CO2–95% air incubator at 37°C for 6 days after infection, at which point all cultures were sampled for supernatant RT activity. The supernatant was clarified, and the viral particles were then pelleted at 40,000 rpm for 30 min by using a rotor and suspended in virus-disrupting buffer. The RT assay was performed in 96-well microdilution plates by using (rA)n. · (dT)12–18 as the template primer. The RT results were expressed in disintegrations per minute of originally clarified supernatant. 3′-azido-3′-deoxythymidine (AZT) was employed as positive control.
Cytotoxicity test
Vero cells.: Cytotoxicity was evaluated by incubating Vero cell monolayers with complete medium containing various dilutions of sample for 72 h at 37°C. Then, cell cytotoxicity was examined by microscopic observation.
PBMCs.: Human PBM cells (5 × 104 cells per well) were seeded in 96-well plates in the presence of increasing concentrations of the test compound and incubated in an incubator at 37°C with 5% CO2. After a 5-day incubation, cell viability was measured using the CellTiter 96 Aqueous One Solution cell proliferation assay (Promega, Madison, WI, USA).
Results and Discussion
Previous chemical studies of A. gomezianus. revealed its phenolic constituents as flavonoids and stilbenoids (Likhitwitayawuid et al., Citation2000; Likhitwitayawuid & Sritularak, Citation2001; Hakim et al., Citation2002). Phenolics found in M. pallidus. are phloroglucinol derivatives (Supudompol et al., Citation2004; Likhitwtaywuid & Supudompol, Citation2005). T. trifolia. is known to produce coumarins as well as a limonoid, an alkaloid, and carotenones (Yokoyama & White, Citation1968; Yokoyama & Guerrero, Citation1970; Dreyer et al., Citation1972; de Silva et al., Citation1981; Abaul et al., Citation1994; Ruangrungsi et al., Citation1994). Our re-investigation of the constituents of the leaves of T. trifolia. yielded 13 coumarins (13–25). Compounds 15–17, 21, 22, and 24 were not found in previous investigations. For antiviral activity studies, all of the phenolics obtained from A. gomezianus., M. pallidus., and T. trifolia. (1–25) were subjected to anti-HSV and/or anti-HIV activity evaluation. Assays were conducted according to previously published protocols for HSV-1 and HSV-2 (Likhitwitayawuid et al., Citation2005) and HIV-1 (Schinazi et al., Citation1990), with acyclovir and AZT as positive controls, respectively. The results are summarized in . It can be seen that the most potent antiviral phenolics in this study are the phloroglucinol dimers 11 and 12, that were obtained from M. pallidus., showing strong activity against both HSV and HIV. However, their antiviral activity seemed to be accompanied by toxicity, as indicated by their IC50 values in Vero cells and PBMCs. Prior to this study, dimeric phloroglucinols from Mallotus japonicus. have been studied for HIV-RT inhibitory activity (Nakane et al., Citation1991). As for the phenolics from A. gomezianus., flavones 1–5 showed appreciable activity against HIV, but their inherent toxicity against PBMCs was also observed. In fact, anti-HIV-RT activities of flavones have long been recognized (Higuchi etal., Citation1991; Vlietnick et al., Citation1998; Du et al., Citation2003). Recently, natural coumarins have received much attention due to their potent anti-HIV activity observed in preclinal and clinical studies (Flavin et al.; Citation1996; Yu et al., Citation2003). This prompted us to examine the coumarins obtained from our chemical study of T. trifolia.. However, it was found that most of them were devoid of antiviral activity, except for 13, 16, and 24, which exhibited modest anti-HIV potential.
Table 1.. Antiviral activity of compounds from A. gomezianus., M. pallidus., and T. trifolia..
When the structures of the active compounds were compared, it could be seen that all, except for 13, 16, and 24, share the bis-hydroxyphenyl structure as their common attribute. Flavones 1–5 each contain two OH-bearing aromatic rings connected through a three-carbon bridge, whereas dimeric phloroglucinols 11,12 are bis-hydroxyphenyls with one-carbon linkage. Recently, bis-hydroxyphenyls with two-carbon bridge such as the stilbenes resveratrol and oxyresveratrol (26 and 27) have been reported to exhibit anti-HSV and anti-HIV activity (Docherty et al., Citation2004; Likhitwitayawuid et al., Citation2005). Thus, it seems that natural bis-hydroxyphenyls with a connecting bridge composed of 1 to 3 carbon atoms (i.e., structure 28) tend to possess anti-HSV and/or anti-HIV activity. It should be mentioned that although the antiviral flavonoids in this study show recognizable cytotoxicity, a chalcone possessing anti-HIV activity with minimal toxicity has been reported (Wu et al., Citation2003). Thus, it appears that bis-hydroxyphenyls might be an attractive target for structure-activity relationship studies. Bis-hydroxyphenyl analogues with suitable linkage, appropriate substituents, and correct spatial arrangement may render new antiviral drugs of the non-nucleoside type.
Acknowledgments
B. Supudompol and B. Sritularak thank the Thailand Research Fund (TRF) for Royal Golden Jubilee Ph.D. scholarships. K.L. is grateful to the TRF (Grant No. DB64880002) for generous financial support.
References
- Abaul J, Philogène E, Bourgeois P, Poupat C, Ahond A, Potier P (1994): Contribution à la connaisssance des Rutacées Américaines: Étude des feuilles de Triphasia trifolia.. J Nat Prod 57: 846–848. [CSA]
- Basnet P, Kadota S, Manandhar K, Manandhar MD, Namba T (1993): Constituents of Boenninghausenia albiflora.: Isolation and identification of some coumarins. Planta Med 59: 384–386. [CSA]
- Bergendorff O, Dekermendjian K, Nielsen M, Shan R, Witt R, Ai J, Sterner O (1997): Furanocoumarins with affinity to brain benzodiazepine receptors in vitro.. Phytochemistry 44: 1121–1124. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chen IS, Lin YC, Tsai IL, Teng CM, Ko FN, Ishikawa T, Ishii H (1995): Coumarins and anti-platelet aggregration constituents from Zanthoxylum schinifolium.. Phytochemistry 39: 1091. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Derdeyn CA, Silvestri G (2005): Viral and host factors in the pathogenesis of HIV infection. Curr Opin Immun 17: 366–373. [CSA], [CROSSREF]
- Dreyer DL, Pickering MV, Cohan P (1972): Distribution of limonoids in the Rutaceae. Phytochemistry 11: 705–713. [CSA], [CROSSREF]
- de Silva LB, Herath WHMW, Jennings RC, Mahendran M, Wannigama GP (1981): Coumarins from Triphasia trifolia.. Phytochemistry 20: 2776–2778. [CSA], [CROSSREF]
- Docherty JJ, Smith JS, Fu MM, Stoner T, Booth T (2004): Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antiviral Res 61: 19–26. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Du J, He ZD, Jiang RW, Ye WC, Xu HX, But PPH (2003): Antiviral flavonoids from the root bark of Morus alba. L. Phytochemistry 62: 1235–1238. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Flavin MT, Rizzo JD, Khilevich A, Kucherenko A, Sheinkman AK, Vilaychack V, Lin L, Chen W, Greenwood EM, Pengsuparp T, Pezzuto JM, Hughes SH, Flavin TM, Cibulski M, Boulanger WA, Shone RL, Xu ZQ (1996): Synthesis, chromatographic resolution, and anti-human immunodeficiency virus activity of (+ / −)-calanolide A and its enantiomers. J Med Chem 39: 1303–1313. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fujioka T, Furumi K, Fujii H, Okabe H, Mihashi K, Nakano Y, Mastunaga H, Katano M, Mori M (1999): Antiproliferative constituents from Umbelliferae plants. V. A new furanocoumarin and falcarindiolo furanocoumarin ethers from the root of Angelica japonica.. Chem Pharm Bull 47: 96–100. [PUBMED], [INFOTRIEVE], [CSA]
- Fukuchi K, Sakagami H, Okuda T, Hatano T, Tanuma S, Kitajima K, Inoue Y, Inoue S, Ichikawa S, Nonoyama M (1989): Inhibition of herpes simplex virus infection by tannins and related compounds. Antiviral Res 11: 285–297. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Guiotto A, Rodighiero P, Quintily U, Pastorini G (1976): Isoponcimarin: New coumarin from Poncirus trifoliata.. Phytochemistry 15: 348. [CSA], [CROSSREF]
- Hakim E, Ulinnuha UZ, Syah YM, Ghisalberti EL (2002): Artoindonesianins N and O, new prenylated stilbene and prenylated arylbenzofuran derivatives from Artocarpus gomezianus.. Fitoterapia 73: 597–603. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Higuchi H, Mori K, Kato A, Ohkuma T, Endo T, Kaji H, Kaji A (1991) Antiretroviral activities of anthraquinones and their inhibitory effects on reverse transcriptase. Antiviral Res 15: 205–216. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Imai F, Itoh K, Kishibuchi N, Kinoshita T, Sankawa U (1989): Constituents of the root bark of Murraya paniculata. collected in Indonesia. Chem Pharm Bull 37: 119–123. [CSA]
- Jensen MM, Wright DN, Robison RA (1997): Microbiology for the Health Sciences, London, Prentice-Hall.
- Kinoshita T, Wu JB, Ho FC (1996): Prenylcoumarins from Murraya paniculata. var. omphalocarpa. (Rutaceae): The absolute configuration of sibiricin, mexoticin and omphamurin. Chem Pharm Bull 44: 1208–1211. [CSA]
- Likhitwitayawuid K, Sritularak B (2001): A new dimeric stilbene with tyrosinase inhibitiory activity from Artocarpus gomezianus.. J Nat Prod 64: 1457–1459. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Likhitwitayawuid K, Sritularak B, Benchanak K, Lipipun V, Mathew J, Schinazi RF (2005): Phenolics with antiviral activity from Millettia erythrocalyx. and Artocarpus lakoocha.. Nat Prod Res 19: 177–182. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Likhitwitayawuid K, Sritularak B, De-Eknamkul W (2000): Tyrosinase inhibitors from Artocarpus gomezianus.. Planta Med 66: 275–277. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Likhitwitayawuid K, Supudompol B (2005): A new phloroglucinol dimmer from Mallotus pallidus.. Heterocycles 165: 161–164. [CSA]
- Lin YM, Flavin MT, Schure R, Chen FC, Sidwell R, Barnard DL, Huffman JH, Kern ER (1999): Antiviral activities of biflavonoids. Planta Med 65: 120–125. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nakane H, Arisawa M, Fujita A, Koshimura S, Ono K (1991): Inhibition of HIV-reverse transcriptase activity by some phloroglucinol derivatives. FEBS Lett 286: 83–85. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ngadjui BT, Ayafor JF, Sondengam BL, Connoly JD (1989): Prenylated coumarins from the leaves of Clausena anisata.. J Nat Prod 52: 243–247. [CSA], [CROSSREF]
- Ruangrungsi N, Lange GL, Organ MG (1994): Coumarins from the leaves of Triphasia trifolia.. Fitoterapia 65: 179–180. [CSA]
- Schinazi RF, Sommadossi JP, Saalmann V, Cannon DL, Xie MW, Hart GC, Smith GA, Hahn EF (1990): Activity of 3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother 34: 1061–1067. [PUBMED], [INFOTRIEVE], [CSA]
- Supudompol B, Likhitwitayawuid K, Houghton PJ (2004): Phloroglucinol derivatives from Mallotus pallidus.. Phytochemistry 65: 2589–2594. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vlietnick AJ, de Bruyne T, Apers S, Pieters LA (1998): Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med 64: 97–109. [CSA]
- Wu TS (1981): Ophamurin—a new coumarin from Murraya omphalocarpa.. Phytochemistry 20: 178–179. [CSA], [CROSSREF]
- Wu JH, Wang XH, Yi YH, Lee KH (2003): Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos.. Bioorg Med Chem Lett 13: 1813–1815. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yokoyama H, White MJ (1968): Citrus carotenoids-VIII. Phytochemistry 7: 1031–1034. [CSA], [CROSSREF]
- Yokoyama H, Guerrero HC (1970): Triphasiaxanthin, a new carotenone. J Org Chem 35: 2080. [CSA], [CROSSREF]
- Yu D, Suzuki M, Xie L, Morris-Natschke SL, Lee KH (2003): Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med Res Rev 23: 322–345. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]