Abstract
The aim of the current study was to evaluate the α.-tocopherol content and to investigate the antioxidant capacities of the extracts prepared from the leaves of Ficus carica. L. (Moraceae). The antioxidant capacities of the extracts were evaluated by the phosphomolibdenum spectrophotometric method. α.-Tocopherol content was determined by using a high-performance liquid chromatography (HPLC)-UV method. Total flavonoid content was determined by using the aluminium chloride method. Total phenol content was estimated by a modified colorimetric method using Folin-Ciocalteau reagent. The results clearly demonstrate that these extracts have antioxidant capacity. Antioxidant capacity results are consistent with total flavonoid and phenol contents. The α.-tocopherol content of the n.-hexane extract was found to be 3.2788%, whereas it was calculated as 0.0570% on the dry-weight basis of the leaves.
Introduction
The medicinal properties of Ficus carica. L. (Moraceae) have been known for centuries. The leaves and the fruits of Ficus carica. are traditionally used as laxative, stimulant, against throat diseases, antitussive, emollient, emmenagogue, and resolvent (Bellakhdar et al., Citation1991; Guarrera et al., Citation2003). A decoction prepared from its leaves is used for hemorroids, whereas an infusion of its fruits can safely be used as a laxative for children. The fresh leaves are dabbed on warts (Baytop, Citation1984).
There are several reports about the special effect of the fig leaves (Ficus carica.) on diabetes (Jouad et al., Citation2001; Leoporatti & Ivancheva, Citation2003). The effect of a decoction of fig leaves on the control of diabetes was studied, and it was shown that short-term hypoglycemic action persisted in humans (Serraclara et al., Citation1998). An aqueous extract of Ficus carica. leaves was found to induce a significant hypoglycemic effect in rats, but the mechanism involved in such an effect was not elucidated (Perez et al., Citation1996).
Despite several experimental studies on the activity of Ficus carica., to our knowledge there are no reports in the literature that determine the active constituents that might be responsible. As protective effects of vitamin E as an antioxidant in diabetes were studied extensively (Baydas et al., Citation2002; Çelik et al., Citation2002); in this study we wanted to evaluate the α.-tocopherol, antioxidant activity, total phenol and flavonoid contents of Ficus carica..
Materials and Methods
Plant material and preparation of extracts
Ficus carica. leaves were collected in May 2003 from Icmeler, Urla, in Izmir. A voucher specimen (no. 1318) of the sample was deposited in the Herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Ege University. The leaves of the plant were dried at room temperature and then reduced to coarse powder. In order to prepare the extracts, 20 g of the sample was separately extracted with n.-hexane, ethyl acetate, ethanol, and methanol, after stirring for 2 days, and then the extraction solvent was evaporated in vacuo. at 40°C. Water extracts of the leaves were prepared by a 2% infusion.
Reagents and solvents
Sulfuric acid, sodium phosphate, ammonium molybdate, ethyl acetate, aluminum chloride, and Folin-Ciocalteau reagent were obtained from Merck. Gallic acid and α.-tocopherol (Sigma) were used as standards. The n.-hexane used for the extraction was purchased from Riedel, whereas the methanol used as eluent in the high-performance liquid chromatography (HPLC) system was purchased from Lab-Scan. Other chemicals used were of analytical grade.
α-Tocopherol identification
The presence of α.-tocopherol in the n.-hexane extract of Ficus carica. leaves was determined by thin-layer chromatography (TLC). Silica plates (5715 Merck) were prewashed in chloroform:methanol (1:1). After drying, the plates were activated at 100°C for 10 min. The extract and the pure standard dissolved in methanol were subjected to TLC using a mixture of cyclohexane:diethyl ether (4:1) as mobile phase. The mobile phase was allowed to run a distance of 100 mm in the saturated tank. The developed plate was left to dry at room temperature, then oven-dried for 15 min at 100°C. The plate was sprayed with 10% CuSO4–phosphoric acid followed by charring at 190°C for 10 min. α.-Tocopherol gave a black spot, and was identified in the extract by comparison of the Rf (0.53) value with that of corresponding pure standard.
HPLC-UV method for α-tocopherol
Standard and sample solutions
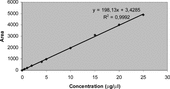
The standard solutions were prepared by dissolving α.-tocopherol in methanol to yield the concentrations of 0.5, 1, 2, 4, 5, 10, 15, 20, and 25 µg/20 µl. The standard solutions were injected on the HPLC column. Concentrations were subjected to regression analyses to calculate the calibration equation and correlation coefficient. Then, the calibration curve was drawn ().
Sample solutions were prepared by dissolving the n.-hexane extracts of the leaves in methanol (10 mg/2.5 ml).Ten microliters of each aliquot was injected on the HPLC column. Each analysis was carried out in triplicate.
The HPLC system consisted of a QuatPump (Hewlett Packard Series 1100), an injector fitted with a 20-µl loop, and a UV detector (HP 1100) set at 292 nm. A Hichrom 5 C18 column (25 cm × 4.6 mm i.d.) was eluted with methanol at a flow rate of 2 ml/min. The column temperature was adjusted to 40°C.
Antioxidant capacity
The spectrophotometric assay for the quantitative determination of antioxidant capacity (TAC) was carried out essentially as described by Prieto et al. (Citation1999). The assay is based on the reduction of Mo (VI) to Mo (V) by the sample analyte and subsequent formation of a green phosphate/Mo (V) complex at acidic pH. An aliquot of 0.1 ml of extracts (1 mg/ml) was combined in an Eppendorf tube with 1 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes were capped and incubated in a thermal block at 95°C for 90 min. After the samples had cooled to room temperature, the absorbance of the aqueous solution was measured at 695 nm against a blank. TAC was determined by comparison with the α.-tocopherol acetate standard calibration curve. The amount of TAC was expressed for extract samples in mM α.-tocopherol acetate equivalent/g dry mass.
Flavonoid content determination
Total flavonoid contents were determined spectrophotometrically in samples according to the German Pharmacopoeia (Deutsches Arzneibuch, Citation1996) method, measuring the flavonoids in AlCl3-complex form from a purified ethyl acetate phase obtained after acid hydrolysis. This is one of the most common analytical procedures applied to flavonoid content determination. The amount of flavonoid was expressed as percent of extracts.
Phenol content determination
The method used for the determination of total phenols using Folin-Ciocalteau reagent was adapted from Mc Donald et al. (Citation2001). Dried samples and standards were prepared in distilled water. Test solutions (samples and standards) of 0.5 ml were added to 4.0 ml of 1 M Na2CO3. Five milliliters of Folin-Ciocalteau reagent (1:10, v/v) were added and the solutions allowed to stand at 45°C for 15 min. Absorbance was measured at 750 nm. The blank consisted of all reagents and solvents without test compounds or standard. The standard was gallic acid prepared in concentrations of 50 to 200 mg/L. This is commonly used as a reference compound. The phenolic concentrations were determined by comparison with the standard calibration curve. Total phenol values were expressed as gallic acid equivalents (mg g−1 dry mass).
Results and Discussion
α-Tocopherol content determined by HPLC-UV method
α.-tocopherol in Ficus carica. leaves was quantitatively determined by a HPLC-UV method. The identification and quantitative determination of α.-tocopherol in the extract were accomplished by a comparison of retention times and areas with that of standard α.-tocopherol and also by spiking the known amount of standard in sample solution. The calibration curve was used for the calculation. The linearity of the HPLC method was checked by injecting nine concentrations of standard α.-tocopherol solutions. Good linearity was achieved in the range 0.5–25 µg/20 µl (r2 = 0.9992). The α.-tocopherol content in the n.-hexane extracts of the leaves of the plant was calculated from the following regression equation of the calibration curve:
where y is the peak area and x is the α.-tocopherol concentration (µg/20 µl). The results of the assay are shown in .
Table 1.. HPLC-UV determination of the α-tocopherol content of fig leaves.
The amount of α.-tocopherol extracted for 100 g of dried leaves of F. carica. was 57 mg. In comparison with previous studies on the α.-tocopherol content of plant leaves, the amount of α.-tocopherol in the dried leaves of Q. ilex., Myrtus communis, Rhamnus alaternus., and Phillyrea angustifolia. were found to be 846, 627, 480, and 442 ppm (Chevolleau et al., Citation1993). Ficus carica. leaves possess a much higher amount of α.-tocopherol than these plants.
The major industrial source of α.-tocopherol is a residue obtained from the distillation of soya bean oil. The content of this compound in the soya bean is only 0.0051–0.0111% (Slover, Citation1983). Because this plant produces a large amount of leaves and their α.-tocopherol is up to 10-times higher than that of soya bean, this plant could be considered as a potential new source of α.-tocopherol. In addition, the HPLC-UV method, found to be suitable for the routine analysis because of its simplicity and sensitivity, can be used for the analysis of α.-tocopherol in other plant extracts.
Antioxidant capacity and total content of phenol and flavonoids
shows the total phenol and flavonoid contents and antioxidant capacities of plant extracts. Much attention has been focused on the protective biochemical function of naturally occurring antioxidants in biological systems and on the mechanism of their action. The TAC method, based on the reduction of Mo (VI) to Mo (V) by sample analyte, was used to measure the amount of antioxidant capacity. The spectrometric assay for the quantitative determination of antioxidant capacity was previously determined for Hypericum. species. The results expressed as nM α.-tocopherol acetate/g dry mass were in the range 4.615–5.483 (Meral, Citation2003). The antioxidant capacity for Ficus carica. leaves ranged from 14.0 to 23.5 mM tocopherol equivalents/g dry mass with the water extract having the highest activity. Thus, it was found that the fig leaves possess substances having high antioxidant activity. The results of this study show that water extract also has the highest total phenol and flavonoid content. There was a correlation between the amount of total phenol and flavonoid contents and the antioxidant capacity. Despite much interest in the antioxidant activity of the leaves of figs, it is uncertain which of the phenols and flavonoids exhibit the greatest antioxidant effect. Further experiments will be conducted to isolate and identify the antioxidant components both qualitatively and quantitatively and assess the mechanisms of activity.
Table 2.. Total flavonoid and phenol contents and antioxidant capacities of the plant extracts.
References
- Baydas G, Canatan H, Türkoğlu A (2002): Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin induced diabetes mellitus. J Pineal Res 32: 225–230. [PUBMED], [INFOTRIEVE], [CSA]
- Baytop T (1984): Therapy with Medicinal Plants in Turkey. No. 3255. Istanbul, Publications of Istanbul University.
- Bellakhdar J, Claisse R, Fleurentin J, Younos C (1991): Repertory of standard herbal drugs in the Moraccan pharmacopoea. J Ethnopharmacol 35: 123–143. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Çelik S, Baydaş G, Yilmaz O (2002): Influence of vitamin E on the leaves of fatty acids and MDA in some tissues of diabetic rats. Cell Biochem Funct 20: 67–71. [CSA], [CROSSREF]
- Chevolleau S, Mallet JF, Debal A, Ucciani E (1993): Antioxidant activity of Mediterranean plant leaves: Occurrence and antioxidant importance of α.-tocopherol. J Am Oil Chem Soc 79: 807–809. [CSA]
- Deutsches Arzneibuch (DAB 10) (1996): Amtliche Ausgabe. Stuttgart, Deutscher Apotheker Verlag.
- Guarrera P (2003): Food medicine and minor nourishment in the folk traditions of central Italy (Marche, Abruzzo and Latinum). Fitoterapia 74: 515–544. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jouad H, Haloui M, Rhiouani H, El Hilaly J, Eddouks M (2001): Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal disease in the North centre region of Morocco (Fez-Boulemane). J Ethnopharmacol 77: 175–182. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Leoporatti ML, Ivancheva S (2003): Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J Ethnopharmacol 87: 123–142. [CSA], [CROSSREF]
- McDonald S, Prenzler PD, Antolovich M, Robards K (2001): Phenolic content and antioxidant activity of olive extract. Food Chem 73: 73–84. [CSA], [CROSSREF]
- Meral G, Konyalıoğlu S (2003): Comparison of total phenol contents and antioxidant capacities of three Hypericum. L. species growing in Turkey. Acta Pharm Turcica 45: 183–185. [CSA]
- Perez C, Dominguez E, Ramiro JM, Romero A, Campillo JE, Torres MD (1996): A study on the glycaemic balance in streptozotocin-diabetic rats treated with an aqueous extract of Ficus carica. (fig tree) leaves. Phytother Res 10: 82–83. [CSA], [CROSSREF]
- Prieto P, Pineda M, Aguilar M (1999): Spectrophotometric quantation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 269: 337–341. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Serraclara A, Hawkins F, Perez C, Dominguez E, Campillo JE, Torres MD (1998): Hypoglycemic action of an oral fig leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract 39: 19–22. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Slover HT (1983): Determination of tocopherols and sterols by capillary gas chromatography. J Am Oil Chem Soc 60: 1524–1528. [CSA]