Abstract
The lack of solubility and the high degree of volatility of essential oils present significant problems to determining the biological effects of these oils. The activity of 10 essential oils and 4 essential oil blends against a Gram-positive bacterium, Staphylococcus aureus., and a yeast, Candida albicans., was compared using a dilution assay and two diffusion methods. The tube dilution assay, using a 0.2% agar solution to provide a stable homogeneous dispersion of oils, was used to measure minimum inhibitory concentrations (MICs). The relative merits of using p.-iodonitro tetrazolium dye (INT) or optical density (OD) in measuring MIC of essential oils also were evaluated. All 14 oils were active against both microorganisms. Thyme, mountain savory, and Turkish oregano were the most active oils against S. aureus. with MICs of 0.31–0.42 µl/ml. Thyme, Turkish oregano, and mountain savory also were the most active against C. albicans. with MICs of 0.31–0.42 µl/ml. The MIC values as determined by INT or OD methods are strongly correlated for both microorganisms, and both give an accurate estimation of MIC. In the disk diffusion assay, thyme, Turkish oregano, Melissa, mountain savory, and the Exodus II (E2) blend were most active against S. aureus.. In the hole-plate assay, the same oils were active against S. aureus. except for Melissa. For C. albicans., thyme, Melissa, mountain savory, Turkish oregano, rosewood, E2 blend, and the T1 blend were most active in the disk diffusion assay. The same oils were active against C. albicans. in the hole-plate diffusion assay except for the T1 blend. Of the methods tested, the tube dilution assay best addresses the solubility and volatility concerns of essential oils. We favor the determination of MIC using INT because of several eliminated steps. Although there were differences in the way in which some essential oils responded in the two diffusion methods, the correlations between the disk and hole-plate diffusion methods were high and yielded comparable results. However, results from the dilution assay were weakly correlated with agar diffusion results. Diffusion assays are useful as a qualitative assessment of biological activity of essential oils but are not appropriate in assessing quantitative effects.
Introduction
Plant-derived essential oils (EOs) are complex mixtures consisting mostly of mono- and sequiterpenes and volatile phenolics (Carson & Riley, Citation1995). Many are known to serve diverse functions in plants such as defenses against fungi, bacteria, and herbivores, in attracting pollinators, as allelopathic agents to reduce the competitive ability of neighboring plants, and in regulating ecosystem processes (Cates, Citation1996). Because of their biological activity, EOs have been explored for their activity against human diseases. Essential oils show marked antibacterial and antifungal effects and have been evaluated as cutaneous and surface antiseptic agents (Salguiero et al., Citation2003), agents against airborne pathogens (Lacoste et al., Citation1996), food preservatives (Choa et al., Citation1998; Lis-Balchin et al., Citation1998), and as acne medications (Lachowicz et al., Citation1998).
Some chemical properties of essential oils, particularly their lack of solubility and high degree of volatility, pose significant problems in accurately assessing biological effects. Methods used to evaluate the biological activity of these oils include qualitative techniques such as disk and hole-plate diffusion assays (Janssen et al., Citation1987; Basset et al., Citation1990) and quantitative dilution methods. Although diffusion assays are fast and simple compared with dilution methods, they are criticized as being unreliable and not suitable for quantitative evaluation of EOs (Janssen et al., Citation1987; Chand et al., Citation1994). The fundamental concern is that diffusion of EO constituents through agar is mutably affected by their physical and chemical properties leading to inappropriate interpretation of their activity. Methods that quantify the microbiological activity of EOs include assays such as agar dilution, tube dilution, and microdilution techniques (Janssen et al., Citation1987). Microtiter-based microdilution assays provide a reliable and efficient method for testing the efficacy of antibiotics. However, our preliminary experiments with EOs in 96-well plates showed that carry-over activity of highly volatile oil components across wells significantly confounded results from such assays. Consequently, an assay using a 96-well plate is unsuitable for rigorously testing EOs against the microorganisms used in this study.
An additional obstacle to testing EOs in any dilution-based assay is the difficulty in obtaining a homogeneous mixture of oils in the test medium. Surfactants, such as Tween 80 (polysorbate 80) (Carson & Riley, Citation1995), Tween 20 (polyoxyethelene-2-sorbatin mono-laurate), dimethyl sulfoxide (Yashphe et al., Citation1979), PEG 400 (polyethylene glycol) in a concentrated sugar solution (Scortichini & Rossi, Citation1991), and ethanol (Briozzo et al., Citation1989; Biondi et al., Citation1993), have been used. However, chemical surfactants may interfere with the activity of EOs against microorganisms resulting in inaccurate estimates of inhibitory properties (Remmal et al., Citation1993). Also, our experience has shown that these surfactants do not produce stable emulsions for the duration of most assays. Based on our preliminary studies, we suggest an 0.2% agar solution to maintain a homogeneous mixture of oils in the media used in the tube dilution assay (Remmal et al., Citation1993).
Objectives
This study reports antimicrobial testing results from a variety of single and blended essential oils using tube dilution and diffusion assays against a Gram-positive bacterium, Staphylococcus aureus., and a yeast, Candida albicans.. Additionally, the tube dilution assay permitted evaluation of the relative merits of using p.-iodonitro tetrazolium dye (INT) or optical density (OD) to measure minimum inhibitory concentrations (MICs) of essential oils. Ten single and four blended oils were used to provide a range of solubilities and volatilities and to provide information on the activities of a set of oils. Correlation analyses were done to determine the degree of agreement between these two methods (Minitab 11.21, Minitab, Inc., State College, PA, USA). Disk and hole-plate diffusion methods were compared in the same manner. Finally, results from the two diffusion assays (i.e., zone diameters) were compared with the results from the tube dilution assay. Because of the previously discussed problems associated with testing EOs in a diffusion-based system, we expected the diffusion and tube dilution methods to differ in their ability to assess relative activities of EOs.
Materials and Methods
Essential oils and microorganisms
Essential oils were obtained from Young Living Essential Oils (Lehi, UT, USA). Fourteen EOs were tested, which included 10 single species preparations and 4 blends (). Staphylococcus aureus. (ATCC 6538P) and Candida albicans. (ATCC 90028) served as the bioassay organisms. All oils and test organisms are archived in the Chemical Ecology Laboratory, Brigham Young University.
Table 1.. Plant species, family, common name, and lot or sample number for single (one species) and blended (two or more species) essential oils used in this study.
Essential oils were steam distilled using low temperature (about 118°C) and pressure (< 5 lb pressure) to ensure minimal changes in the chemical properties of the oils (Young, Citation2003). The oils tested were without solvents of any kind such as acetone and methanol. Factors that influence quality of oils such as weather and soil conditions, plant maturity level, time of harvest, harvest method, time between harvest and distillation, and amount of material distilled at any given time were controlled as closely as possible. Prior to use in these tests, the oils were stored at 5°C to minimize volatilization and change in chemical properties.
Tube dilution technique
To maintain a homogeneous mixture of oils in the test media, an 0.2% (w/v) agar solution (Difco, Bitek; Becton, Dickinson and Company, Sparks, MD, USA) was prepared in Tryptic Soy Broth (TSB; Becton Dickinson and Company, Cockeysville, MD, USA) for S. aureus. and Sabouraud Dextrose Broth (SDB; Becton Dickinson, Sparks, MD, USA) for C. albicans. (Remmal et al., Citation1993). Serial dilutions of EOs were prepared by adding 4 ml of the 0.2% agar solution to a 13-mm culture tube, and 2 ml each to five additional test tubes. To obtain an initial concentration of 10 µl/ml, 40 µl essential oil was added to the first tube. The preparation was vortexed to establish a homogeneous and stable microsuspension of EO. This dispersion was serially diluted by 50% in each of the remaining five tubes. Each dilution was vortexed prior to transferring an aliquot to the next tube creating an EO concentration range from 10 µl/ml (tube 1) to 0.3 µl/ml (tube 6). Two milliliters were removed from the final dilution and discarded. For EO active at or near tube 6, additional dilutions were made in subsequent assays.
All test tubes in the serial dilution along with the three control tubes were inoculated with 20 µl of an overnight culture of the microorganism to be assayed. The inoculated tubes were vortexed and optical density (OD) was measured for all tubes using a spectrophotometer (600 nm, Beckman DU 650; Beckman Coultor, Fullerton, CA, USA). After 24 h of incubation (35°C; shaken at 90 rpm), tubes were vortexed until homogenized and OD was again measured. We calculated percent inhibition as the net change in OD for each tube divided by the average change for controls times 100.
Initially, the EO concentration at which percent inhibition equaled 100 was to be defined as the MIC. However, during incubation some oils caused marked discoloration of the culture media resulting in an underestimation of percent inhibition. This discoloration was accounted for by incubating each oil in uninoculated media for 24 h, measuring the change in turbidity, and adding this correction factor to final OD values.
In order to compare the efficacy of p.-iodonitro tetrazolium dye (INT) and optical density (OD) in measuring MIC, 800 µl of p.-iodonitro tetrazolium violet (INT) (0.2 mg/ml) were added to each tube after incubation and after the final OD measurement was made. The colorless INT solution is reduced by respiratory electron transport chain activity to a purple INT formazan dye. The MIC was determined as the concentration of EO at which no reduction of INT was observed (no coloration developed within 30 min of adding the dye) (Mann & Markham, Citation1998). The tube containing the MIC as well as those bracketing this concentration were streaked onto Tryptic Soy Agar with Dextrose (TSA; Becton Dickinson, Sparks, MD, USA) (Staphylococcus.) and Sabouraud Dextrose Agar (SDA; Becton Dickinson, Sparks, MD, USA) (Candida.) and incubated overnight to verify MIC values. Assays were repeated three times on different days, and MIC is reported as an average of the three values.
Disk and hole-plate diffusion
Diffusion assays were carried out in 150-mm-diameter Petri dishes. Plates were poured with 55 ml of agar media (Mueller Hinton Agar [MHA], [Becton Dickinson, Sparks, MD, USA], S. aureus.; SDA, C. albicans.) and streaked with an overnight culture using a cotton-tipped applicator. Standard Kirby-Bauer disk diffusion tests were performed for each oil (Eloff, Citation1998). For disk diffusion assays, 8-mm paper disks (BBL Blank Paper Discs, Becton Dickinson, Sparks, MD, USA) were impregnated with 15 µl of EO and immediately placed on the agar surface. In the hole-plate diffusion assays, cores were removed from the agar using a 4-mm cork borer, and 15 µl of essential oil was added to the hole.
After 24 h of incubation, a zone of inhibition was interpreted as the area of the agar surface radiating from the disk or the hole that was free of bacterial growth. Two measurements perpendicular to each other were taken from the edge of the paper disk or hole to the outer margin of the zone of inhibition and were averaged. Due to volatility of the oils, only one EO was tested per Petri dish; therefore, each disk represented one replicate. (Assays were repeated three-times, and the average zone size is reported in .)
Results and Discussion
Minimum inhibitory concentrations of essential oils based on the tube dilution method
All 14 oils tested were inhibitory to both microorganisms. Thyme, mountain savory, and Turkish oregano produced the lowest MICs against S. aureus. whether measured by INT or % inhibition (OD) (). The MICs as estimated by INT and OD methods against S. aureus. were highly correlated (r2 = 0.908; ). In dilution assays against C. albicans., thyme, Turkish oregano, and mountain savory also were the most active oils (). Again, the relationship between MIC values as estimated by INT and OD was strongly correlated (r2 = 0.955; ).
Figure 1 Correlation between INT and OD measurements in determining the minimum inhibitory concentration (MIC) of 14 essential oils against Staphylococcus aureus. (A) and Candida albicans. (B).
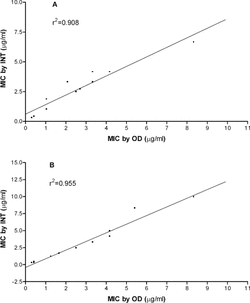
Table 2.. Minimum inhibitory concentration (MIC) of 14 essential oils against Staphylococcus aureus. as determined by the metabolic dye (INT) and % inhibition (OD).
Table 3.. Minimum inhibitory concentration (MIC) of 14 essential oils against Candida albicans. as determined by the metabolic dye (INT) and % inhibition (OD).
For both methods, the day to day variability in MIC range was not more than one dilution removed in this series (Tables and ). However, six of the ranges are broader than one dilution removed and as high as three dilutions removed (e.g., spruce, C. albicans., % inhibition). This degree of variation has been noted in other studies (Carson & Riley, Citation1995; Eloff, Citation1998). Reasons for the observed variability are unknown but might be attributed to nonuniform suspensions of EOs in serial dilutions during the course of the assay and/or oils adhering differentially to polypropylene pipette tips. Recently, our studies have shown some reduction of variation when the dilution series is based on weight to volume concentrations rather than volume to volume as in this study.
Rosewood, spruce and white fir inhibitions were all ranked considerably higher against S. aureus. than against C. albicans. relative to other oils, while the E2 blend was ranked markedly higher against C. albicans. than against S. aureus. (Tables and ). These shifts in ranking of relative activity of these four oils may suggest microbe-specific activity. Because the INT and OD methods are highly correlated, and given the small number of viable cells present at the MIC, we suggest that both methods give an acceptable interpretation of MIC. However, the OD method includes several laborious steps such as spectrophotometric readings and computational analysis of optical densities. These steps are eliminated in the INT method, and therefore we suggest it to be the preferred method.
EO zones of inhibition in agar diffusion assays
In these tests, all EOs were inhibitory to both microorganisms. In the disk diffusion assay, thyme, Turkish oregano, Melissa, mountain savory, and the E2 blend produced the largest zones of inhibition against S. aureus. (). In the hole-plate assay, thyme, Turkish oregano, the E2 blend, and mountain savory were the most active against S. aureus.. For C. albicans., thyme, Melissa, mountain savory, Turkish oregano, rosewood, the E2 blend, and the T1 blend were the most active in the disk diffusion assay (). In the hole-plate assay, thyme, rosewood, Turkish oregano, mountain savory, the E2 blend, and Melissa were the most active against C. albicans..
Table 4.. Zones of inhibition for diffusion assays against Staphylococcus aureus. and Candida albicans..
Melissa showed less activity against both microorganisms in the hole-plate assay when compared with its effects in the disk assay. In the hole-plate assay, Melissa had the fifth largest inhibitory zone against S. aureus. but was the third most active EO in the disk diffusion assay. This discrepancy between diffusion methods is more pronounced in assays against C. albicans.. Melissa oil produced the second largest zone in the disk assay but only the sixth largest in the hole-plate assay.
Uncovered in our studies is a problem not noted in the literature, at least to our knowledge. In preliminary experiments, we found that volatile components of Melissa oil caused reductions in the growth of bacteria in adjacent wells of 96-well microtiter trays. The sixth column of wells (12 total) of a pre-inoculated microtiter tray was loaded with Melissa oil at 1% and 0.05% concentrations (rows 1–4 and 5–8, respectively). Optical density (absorbance at 600 nm) was used to determine relative amounts of bacterial growth between columns. At both concentrations, significant reductions in growth occurred in adjacent wells at least four columns removed from wells in which the EO was applied (p < 0.05; ANOVA, Tukey's family error rate). These results show that dilution assays in a microtiter plate may produce erroneous data and should not be used to test EO for activity against the microorganisms used in this study. Furthermore, the differences observed with Melissa oil in the disk diffusion compared with the hole-plate diffusion assay may be due to highly volatile and inhibitory components (). Preliminary results from experiments where EO-impregnated disks were placed on the lid of inverted plates indicate that Melissa volatiles are highly active against S. aureus.. Differences in inhibition between the two methods may be due to the disk providing more surface area for compounds to volatilize from compared with the hole-plate assay.
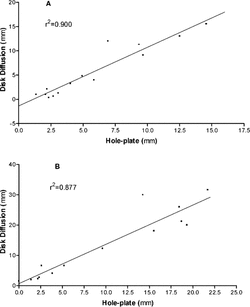
As indicated above, there are inherent differences in the way in which some EOs respond to these two diffusion methods. This is especially true for C. albicans., for which all zones of inhibition were lower in the hole-plate assay compared with the disk method. In spite of this, overall correlations between the methods remained strong for both S. aureus. and C. albicans. (r2 = 0.90, ; r2 = 0.877, , respectively), indicating that the methods are yielding comparable results.
Figure 2 Correlation between disk and hole-plate diffusion assay results for Staphylococcus aureus. (A) and Candida albicans. (B).
In general, inhibition zones in assays against C. albicans. were twice those observed in S. aureus. assays; this is especially true for the disk diffusion assay. Perhaps the extracts are more active against C. albicans., but this phenomenon may be due to chemical or physical differences in the properties of SDA and MHA media used to culture the microbes. This illustrates a common criticism of diffusion assays (Yasphe et al., Citation1979; Chand, et al., Citation1994), and especially when testing essential oils, as they are highly nonpolar. Inhibition data generated from diffusion assays may not truly represent the activity of an EO against a microbe. Even in a consistent medium, differences in polarities and other properties of essential oils may significantly confound results. For example, in disk assays Turkish oregano is the second most active extract against S. aureus. but the fourth most active against C. albicans. (). Several oils show differences in activity between S. aureus. and C. albicans.. Whether these differences are due to specificity of activity or due to differences in the affinity of oils to the agar media is unresolved.
Diffusion and dilution methods compared
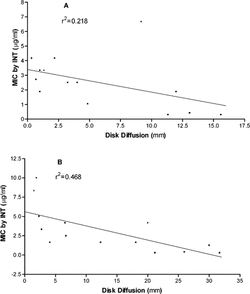
To assess how well quantitative assessments of the MICs using the dilution assay agreed with agar diffusion results, regressions were made between MIC (using INT data) and disk diffusion zones (). For S. aureus., the relationship is weak (r2 = 0.218) and not statistically significant (p = 0.09; ). However, it is important to note that this relationship is affected by the results of blend E2. Removing it from the analysis improved the correlation (r2 = 0.468). Blend E2 was low in activity in the dilution assay () but produced the fifth largest zone of inhibition in the diffusion assay. Reasons for this are unclear. For C. albicans., there was a statistically significant relationship (p = 0.007; ) between the MICs as determined by INT and the disk diffusion method, but the relationship is weak (r2 = 0.468). Regressions between MIC data and hole-plate diffusion data gave similar results (data not shown). Overall, these results illustrate the limitation of diffusion assays. Although they are fast and inexpensive, results should not be interpreted as quantitative in the assessment of antimicrobial activity of EOs.
Conclusions
The tube dilution method, as modified in this paper, is an efficacious procedure for estimating MIC values for essential oils. For all oils, a solution of 0.2% agar added to the growth medium achieved a stable dispersion of the oils in the aqueous media. The tube dilution method also removes the carry-over problem that may occur when 96-well microtiter trays are used to test volatile chemical compounds. Optical density measurements result in a determination of percent inhibition, which can then be used to calculate a MIC. The MIC determined from the metabolic dye INT also resulted in accurate measurements of the minimum inhibitory concentration. Furthermore, these two methods yielded comparable results. We conclude that both methods for assessing MICs of EOs are acceptable, and, depending on the availability of resources, either could be used interchangeably. However, because of the additional steps and computational analyses involved in the OD method, we favor the determination of a MIC using the metabolic dye INT. Although diffusion assays are useful as a qualitative measure of biological activity of EOs, they are not appropriate in assessing useful quantitative effects of these compounds.
Additionally, this study indicates that essential oils are active against microbes. All 14 essential oils in this study showed significant inhibitory activity against both microorganisms. Thyme, mountain savory, and Turkish oregano were the most active essential oils against both the bacteria and the yeast. As determined by the tube dilution assay, four of the essential oils showed microbe-specific activity. Rosewood, spruce, and white fir were more active against S. aureus. than against C. albicans., while the blend E2 (Exodus II) was more inhibitory to C. albicans..
References
- Basset IB, Pannowitz DL, Barnetson RS (1990): A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Med Australia 153: 455–458. [CSA]
- Biondi D, Cianci P, Geraci C, Ruberto G (1993): Antimicrobial activity and chemical composition of essential oils from Sicilian aromatic plants. Flav Fragr J 8: 331–337. [CSA]
- Briozzo J, Nunez L, Chirife J, Herszage L, D'Auino M (1989): Antimicrobial activity of clove oil dispersed in a concentration sugar solution. J Appl Bacteriol 66: 69–75. [PUBMED], [INFOTRIEVE], [CSA]
- Carson CF, Riley TV (1995): Antimicrobial activity of the major components of the essential oil of Melalueca alternifolia.. J Appl Bacteriol 78: 264–269. [PUBMED], [INFOTRIEVE], [CSA]
- Cates RG (1996): The role of mixtures and variation in the production of terpenoids in conifer-insect-pathogen interactions. Rec Adv Phytochem 30: 179–216. [CSA]
- Chand S, Lusunzi I, Veal DA, Williams LR, Karuso P (1994): Rapid screening of the antimicrobial activity of extracts and natural products. J Antibiot (Tokyo) 47: 1295–1304. [CSA]
- Choa SC, Young DG, Oberg CJ (1998): Effect of a diffused essential oil blend on bacterial bioaerosols. J Essen Oil Res 10: 517–523. [CSA]
- Eloff JN (1998): A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Plant Med 64: 711–713. [CSA]
- Janssen AM, Scheffer JJ, Baerheim-Svendsen A (1987): Antimicrobial activities of essential oils. A 1976–1986 literature review on possible applications. Pharm Week Sci Ed 9: 193–197. [CSA]
- Lachowicz K, Jones G, Briggs D, Beinvenu F, Wan J, Wilcock A, Coventry, M. (1998): The synergistic preservative effects of essential oils of sweet basil (Octimum basilicum. L.) against acid-tolerant food microflora. Lett Appl Microbiol 26: 209–214. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lacoste E, Chaumont JP, Mandin D, Plumel MM, Matos FJ (1996): Antiseptic properties of essential oil of Lippia sidoides. Cham. Application to the cutaneous microflora. Ann Pharm Fr 54: 228–230. [PUBMED], [INFOTRIEVE], [CSA]
- Lis-Balchin M, Buchbauer G, Hirtenleher T, Resch M (1998): Antimicrobial activity of Pelargonium. essential oils added to a quiche filling as a model food system. Lett Appl Microbiol 27: 207–210. [PUBMED], [INFOTRIEVE], [CSA]
- Mann CM, Markham JL (1998): A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84: 538–544. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Remmal A, Bouchikhi T, Tantaoui-Elaraki A, Ettayebi M (1993): Inhibition of antibacterial activity of essential oils by Tween 80 and ethanol in liquid medium. J Pharm Belgique 48: 352–356. [PUBMED], [INFOTRIEVE], [CSA]
- Salguiero LR, Cavaleiro C, Pinto E, Pina-Vaz C, Rodrigues AG, Palmeira A, Tavares C, Costa-de-Oliveira S, Goncalves MJ, Marinez-de-Oliveira J (2003): Chemical composition and antifungal activity of the essential oil of Origanum virens. on Candida. species. Plant Med 69: 871–874. [CSA], [CROSSREF]
- Scortichini M, Rossi MP (1991): Preliminary in vitro. evaluation of the antimicrobial activity of terpenes and terpenoids towards Erwinia amylovora. (Burrill) Winslow. J Appl Bacteriol 71: 109–112. [CSA]
- Yashphe J, Segal R, Breuer A, Erdreich-Naftali G (1979): Antibacterial activity of Artemisia herbaalba.. J Pharm Sci 68: 924–925. [PUBMED], [INFOTRIEVE], [CSA]
- Young DG (2003): Essential Oils: Integrative Medical Guide. Salt Lake City, Essential Science Publishing, p. 610.