Abstract
Methanol extracts of three Caloplaca. species showed broad-spectrum antifungal and antibacterial properties against some human and plant pathogens. Fractionation with methylene chloride and isolation of pure anthraquinone derivatives substantially increased the level of antimicrobial activity. Eight anthraquinone derivatives were isolated from the three species. 1-O.-Methylparietin and 8-O.-methylparietin were isolated from lichens for the first time. Minimum inhibitory concentrations for the extracts were found to be 80–320 µg/ml for bacteria and 40–320 µg/ml for fungi, and the pure anthraquinones range from 20 to 320 µg/ml for bacteria and from 20 to 160 µg/ml for fungi.
Introduction
Lichens produce a diverse range of primary and secondary metabolites (Hale, Citation1983). Slow growth, and often harsh living conditions, makes production of protective compounds a necessity for lichens. The secondary metabolites possess a broad spectrum of biological activities including antibacterial (Lauterwein et al., Citation1995; Yamamoto et al., Citation1998), enzyme inhibition (Matsubara et al., Citation1997), antifungal (Proksa et al., Citation1996; Shahi et al., Citation2001), analgesic and antipyretic (Okuyama et al., Citation1994), antitumor (Kupchan & Kopperman, Citation1975), and antiviral (Cohen et al., Citation1996) activity.
Caloplaca lactea., C. citrina., and C. schaereri. are lichens belonging to the family Teloschistaceae. Previously, only four different anthraquinone derivatives from these three lichen species have been detected by thin-layer chromatography (TLC) and lichen mass spectrometry (LMS) but not isolated (Santesson, Citation1970).
Anthraquinones are components in many medicines of plant origin because they possess antibacterial, anti inflammatory, antitumor, purgative, astringent, antiviral (Cyong et al., Citation1987; Muzychkina, Citation1998), and antifungal properties (Agarwal et al,. Citation2000; Manojlovic et al., Citation2001). Lichens produce some characteristic anthraquinone derivatives, which have yet to be found in higher plants (Santesson, Citation1970; Nakano et al., Citation1972; Steglich & Reininger, Citation1972; Søchting, Citation1997Citation2001; Rosso et al., Citation2003).
Materials and Methods
Lichen species. studied
The lichens of Caloplaca lactea. (Massal.) Zahlbr. and C. citrina. (Hoffm.) Th.Fr. were collected in July 2000 from Gledic Mountain (Serbia and Montenegro), and C. schaereri. (Arnold) Zahlbr. was collected in July 1998 from Durmitor Mountain (Serbia and Montenegro). These Caloplaca. species were identified by S. Savic, Natural History Museum, Belgrade, Serbia and Montenegro, where voucher specimens have been deposited (C. lactea., BEO 1446; C. citrina., BEO 1444; C. schaereri., 1445).
General experimental procedures
UV-Vis spectra were recorded on a Perkin Elmer Lambda 35 UV-Vis spectrometer, using ethanol as the solvent, and mass spectra was determined with a Finigan-MAT 8230 instrument. 1H NMR spectra were recorded on a Varian Gemini (200 MHz) instrument in CDCl3 using TMS as an internal standard. Melting points (m.p.) were determined on a Kofler hot-stage apparatus and are uncorrected. Silica gel (Merck, 0.063–0.02 mm) and Sephadex LH-20 were used for column chromatography. Preparative layer chromatography was performed on 20 × 20 cm glass plates coated with 1 mm of silica (Merck, Kieselgel GF254 applied as a suspension in H2O).
Preparation of lichen extracts and active principles
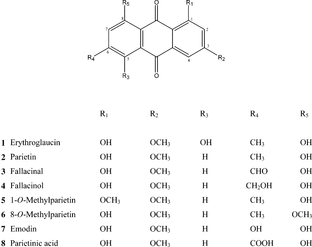
Methanol extracts of three lichen species were prepared in a Soxhlet apparatus using 20 g of each lichen in 200 ml solvent for 6 h. These extracts were used for antimicrobial and chemical determination. The extracts were analyzed by TLC on silica gel using benzene and benzene-acetone mixtures as eluent, detected under UV 254 nm, and visualized by spraying with a methanolic solution of magnesium acetate along with standard markers (parietin, fallacinal, fallacinol, emodin, erythroglaucin, and parietinic acid). The methanol extracts were fractionated with methylene chloride, and after TLC determination metabolites from the methylene chloride fractions were separated on a silica gel column using benzene, benzene-acetone (20:1, 10:1, and 5:1), and acetone as eluents. Finally, the column was washed with a mixture of ethyl acetate/methanol/acetone. The results of chemical analysis are shown in . Eight structurally different multisubstituted anthraquinone derivatives were isolated ().
Table 1.. Distribution of anthraquinone derivatives in three Caloplaca..
Spectral data for new lichen metabolites
8-O.-Methylparietin: yellow needles; m.p. 192–194°C; UV-Vis (ethanol): (λ log ε): 223 (3.96), 268 (4.55), 285 (4.60) and 428 (3.86). Mass spectrum m./z. (%): 298 (M+, 100 %), 280 (39), 269 (20), 252 (44), 149 (75). 1H NMR (CDCl3): δ: 2.51 (3H, s, Me), 3.92 (3H, s, OMe), 4.06 (3H, s, OMe), 6.71 (1H, d, J.-2.5 Hz, H-2), 7.12 (1H, br s, H-7), 7.31 (1H, d, J.-2.5 Hz, H-4), 7.77 (1H, br s, H-5), and 13.32 (1H, s, OH-1).
1-O.-Methylparietin: yellow needles; m.p. 212–213°C; UV-Vis (ethanol): (λ log ε) 270 (4.18), 282 (4.20) and 426 (3.85). Mass spectrum m./z. (%): 298 (M+) (100), 281 (70), 280 (97), 269 (84), 252 (78). 1H NMR (CDCl3): δ 2.44 (3H, s, Me), 3.99 (3H, s, OMe), 4.04 (3H, s, OMe), 6.80 (1H, d, J.-2.5 Hz, H-2), 7.08 (br s, 1H, H-7), 7.48 (d, J.-2.5 Hz, 1H, H-4), 7.58 (1H, br s, H-5), and 13.11 (1H, s, OH-8).
Microorganisms
The bacterial strains of Bacillus cereus., Bacillus subtilis., Escherichia coli., Staphylococcus aureus., and Pseudomonas aeruginosa. and fungal strains of Candida albicans., Trichophyton mentagrophytes., Epidermophyton floccosum., and Trichoderma harzianum. were used for the antimicrobial investigations. All fungi and bacteria were obtained from the culture collection maintained in the Department of Biology, Faculty of Science, University of Kragujevac, Serbia and Montenegro.
Antimicrobial assays
Antimicrobial activity in vitro. was studied by determination of the minimum inhibitory concentrations (MICs) using a twofold broth dilution method as previously described in Lichenologist. (Manojlovic et al., Citation2002). Anthraquinones and lichen extracts at concentrations 10, 20, 40, 80, 160, and 320 µg/ml were used. Inoculation was carried out with overnight cultures of the strains, to a final inoculum size of 105 CFU/ml for the bacteria and 104 CFU/ml for the fungi. MICs were read after 24 h incubation at 37°C for the bacteria and 48 h at 30°C for the fungi and were taken equal to the lowest concentration of the compounds where no visually observable growth.
Results and Discussion
Air-dried Caloplaca lactea., C. citrina., and C. schaereri. were extracted with methanol, and all the extracts (ME) obtained were tested for antibacterial and antifungal activity. The lichen extracts exerted varying levels of antimicrobial effects against microorganisms. MIC values of the tested extracts are shown in . MIC values for bacteria are from 160 to 320 µg/ml. C. lactea. methanol extract showed greater antimicrobial effects against all microbial species than other lichen methanol extracts. On the other hand, all the tested extracts did not show antibacterial effects on Gram-negative Escherichia coli. and Pseudomonas aeruginosa. (MIC > 320 µg/ml). Testing of the methanol extracts on four fungi showed the MICs to be 80–320 µg/ml. Fractionation with methylene chloride increased the level of activity considerably. In this testing, the Gram-positive bacteria were more sensitive than Gram-negative bacteria, and the MIC values for them ranged from 80 to 320 µg/ml depending on the extract. The MIC values for fungi were 40–320 µg/ml. The results showed that methylene chloride fractions of C. lactea. and C. schaereri. exhibited the greatest activity toward Trichoderma harzianum. (MIC 40 µg/ml). As in the case of methanol extracts, methyene chloride fractions were not effective on Gram-negative bacteria Escherichia coli. and Pseudomonas aeruginosa. even at higher concentrations (MIC > 320 µg/ml).
Table 2.. Minimum inhibitory concentration (MIC) values (µg/ml) for antibacterial and antifungal activity of the methylene chloride (MH) and methylene (ME) extracts of C. lactea., C. citrina., and C. schaereri..
After examining the antibacterial and antifungal activity of the extracts, secondary anthraquinone metabolites were isolated and identified from the extracts. These were suspected to be carriers of exhibited activity. Methylene chloride fractions contain the same anthraquinone derivatives as well as methanol extracts of the corresponding lichen. Methanol extracts contained polyols (mainly d-mannitol and d-arabitol) besides anthraquinones and some other minor compounds, whereas in the methylene chloride fractions polyols could not be found. Anthraquinones were separated on a silica gel column using benzene, benzene-acetone (20:1, 10:1, and 5:1), and acetone as eluents. Four anthraquinone derivatives from the C. citrina. extract—parietin, fallacinal, fallacinol, and emodin—were isolated. Parietin was the primary constituent of the three extracts. Chromatographic purification of the methylene chloride extract of C. schaereri. produced parietin, fallacinal, fallacinol, emodin, and parietinic acid. Their identities were established by comparison of the m.p. UV-Vis, 1H NMR, and mass spectral data with that of the known compounds (Krivoshchekova et al., Citation1981; Yoshimura et al., Citation1994; Manojlovic et al., Citation2001; Rosso et al., Citation2003). Chemical analysis of C. lactea., whose extract exhibited the most significant level of antibacterial and antifungal activity, showed anthraquinone derivatives not found in C. citrina. and C. schaereri.. Seven anthraquinone derivatives were identified in the methylene chloride extract using column and preparative layer chromatography with different eluents. Besides parietin, fallacinal, fallacinol, emodin, and eryhroglaucin, two O.-alkylated parietin derivatives (not previously found in Caloplaca.) were isolated from the C. lactea. extract. The identities of these compounds were established by m.p., 1H NMR, UV and mass spectra. This is the first report of parietin ethers that could have been formed by methylation of hydroxyl groups of parietin (at C-1 and C-8), similar to methylation of the hydroxyl group of emodin (at C-6). These anthraquinones were synthesized earlier from emodin derivatives (Savard & Brassard, Citation1984) and cycloaddition of diene and napthaquinones (Cameron & Crossley, Citation1977).
After isolation and identification, the pure anthraquinones were tested on the same species of bacteria and fungi, as in the case of the extracts. The results showed a more significant level of antimicrobial activity. The anthraquinones exhibited considerable antibacterial effects on the species tested with MICs ranging from 20 to 320 µg/ml. Fallacinol showed the best effects againstStaphylococcus aureus. (MIC 20 µg/ml) and Bacillus subtilis. (MIC 40 µg/ml), from which it may be concluded that an anthraquinone with a hydroxymethyl group in position C-3 shows greater antibacterial activity than other derivatives. 1-O.-methyl parietin and 8-O.-methyl parietin showed antibacterial effect toward Gram-positive species Bacillus cereus., Bacillus subtilis., and Staphylococcus aureus. with MIC 40–160 µg/ml.
None of the compounds tested showed inhibitory activity toward Gram-negative Escherichia coli. and Pseudomonas aeruginosa. (MIC > 320 µg/ml). Examination of the results of the antifungal activity showed significant inhibition against Candida albicans., Trichophyton mentagrophytes., Epidermophyton floccosum., and Trichoderma harzianum.. These anthraquinones demonstrated greater antifungal activity than crude extracts and metabolites of some anthraquinone-containing medical plants (Agarwal et al., Citation2000). As shown in , MICs were 10–160 µg/ml. Among the tested compounds, fallacinol, which is identified as a constituent of all three species examined, showed the most potent antifungal effect on Trichoderma harzianum. (MIC 10 µg/ml).
Table 3.. Minimum inhibitory concentration (MIC) values (µg/ml) for antibacterial and antifungal activity of the anthraquinones from C. lactea., C. citrina., and C. schaereri..
These results show that the anthraquinone structure plays a significant role in expressing antibacterial and antifungal activity, but the presence of both hydroxyl groups (at C-1 and C-8) is not necessary for exhibiting this activity. In the light of the above, it is concluded that C. lactea., whose extract exhibits the highest level of activity, is chemically different from the other Caloplaca. that were investigated (Santesson, Citation1970; Søchting, Citation1997Citation2001) and contains the greatest number of structurally different anthraquinones. The O.-alkylated parietin derivatives also show significant antimicrobial activity, which confirms the hypothesis that lichens represent an important source for the production of new antimicrobial agents.
Acknowledgment
The authors acknowledge financial support by the Ministry of Science, Technology and Development of Serbia (grant no. 0295 and no. 1740).
References
- Agarwal KS, Sudhik S Singh, Verma S, Kumar S (2000): Antifungal activity of anthraquinone derivatives from Rheum emodi.. J Ethnopharmacol 72: 43–46. [PUBMED], [INFOTRIEVE], [CSA]
- Cameron DW, Crossley MJ (1977): Synthesis of emodin methyl ethers. Aus J Chem 30: 1161–1165. [CSA]
- Cohen PA, Hudson JB, Towers GHN (1996): Antiviral activities of anthraquinones, bianthrones and hypericin derivatives from lichens. Experientia 52: 180–183. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cyong JC, Matsumoto T, Arakawa K, Kiyohara H, Yamoda H, Otsuka Y (1987): Antibacteroides fragilis substance from rhubarb. J Ethnopharmacol 19: 279–283. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hale ME (1983): The Biology of Lichens, 4th ed. London, Edward Arnold.
- Krivoshchekova OE, Maximov OB, Mischenko NP, Stepanenko LS (1981): Anthraquinones of the lichen Xanthoria aureola. and X. ulophiloides.. Khim Prir Soed 1: 96–97. [CSA]
- Kupchan SM, Kopperman HL (1975): l-Usnic acid: Tumor inhibitor isolated from lichens. Experientia 31: 625–626. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lauterwein M, Oethinger M, Belsner K, Peters T, Marre R (1995): In vitro. activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (−)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob Agent Chemother 39: 2541–2543. [CSA]
- Manojlovic NT, Solujic S, Sukdolak S, Vucetic J (2001): Antifungal activity of extract and natural anthraquinones isolated from Frangula alnus. Mill. and two species of the lichen genus Xanthoria.. Arch Pharm 1–2: 43–47. [PUBMED], [INFOTRIEVE], [CSA]
- Manojlovic NT, Solujic S, Sukdolak S (2002): Antimicrobial activity of an extract and anthraquinones from Caloplaca schaereri.. Lichenologist 34: 83–85. [CSA], [CROSSREF]
- Matsubara H, Kinoshita K, Koyama K, Yang Ye, Takahashi K, Yoshimura I, Yamamoto Y, Miura Y, Kinoshita Y (1997): Inhibitory effect of lichen metabolites and their synthetic analogues on melanin biosynthesis in cultured B-16 melanoma cells. Nat Prod Sci 4: 161–169. [CSA]
- Muzychkina RA (1998): Natural Anthraquinones, Biological and Physicochemical Properties. Moscow, House Phasis.
- Nakano H, Komiya T, Shibata S (1972): Anthraquinones of the lichens of Xanthoria. and Caloplaca. and their cultivated mycobionts. Phytochemisty 11: 3505–3508. [CSA], [CROSSREF]
- Okuyama E, Umeyama K, Yamazaki M, Kinoshita Y, Yamamoto Y (1994): Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Vain. Planta Med 61: 113–115. [CSA]
- Proksa B, Sturdikova M, Pronayova N, Liptaj T (1996): (−)-Usnic acid and its derivatives. Their inhibition of fungal growth and enzyme activity. Pharmazie 51: 195–196. [CSA]
- Rosso ML, Bertoni MD, Adler MT, Maier MS (2003): Anthraquinones from the cultured lichen mycobionts of Teloschistes exilis. and Caloplaca erythrantha.. Biochem Sys Ecol 31: 1197–1200. [CSA], [CROSSREF]
- Santesson J (1970): Anthraquinones in Caloplaca.. Phytochemistry 9: 2149–2166. [CSA], [CROSSREF]
- Savard J, Brassard P (1984): Reactions of ketone acetals-14′. Tetrahedron 40: 3455–3464. [CSA], [CROSSREF]
- Shahi SK, Shukla AC, Dikshit A, Uperti DK (2001): Broad-spectrum antifungal properties of the lichen Heterodermia leucomela.. Lichenologist 33: 177–179. [CSA], [CROSSREF]
- Søchting U (1997): Two major anthraquinone chemosyndromes in Teloschistaceae.. Bibl Lichen 68: 135–144. [CSA]
- Søchting U (2001): Chemosyndromes with chlorinated anthraquinones in the lichen genus Caloplaca. Bibl Lichen. 78: 395–404. [CSA]
- Steglich W, Reininger W (1972): Anthrachinon-pigment aus Dermocybe cinnabarina. (Fr.) Wunsche. Chem Ber 105: 2922–2927. [PUBMED], [INFOTRIEVE], [CSA]
- Yamamoto Y, Kinoshita Y, Matsubara H, Kinoshita K, Koyama K, Takahashi K, Kurokawa T, Yoshimura I (1998): Screening of biological activities and isolation of biological-active compounds from lichens. Recent Res Phytochem 2: 23–34. [CSA]
- Yoshimura I, Kurokawa T, Kinoshita Y, Yamamoto Y, Miyawaki H (1994): Lichen substances in cultured lichens. J Hat Bot Lab 76: 249–261. [CSA]