Abstract
In this work, we studied the effects of lonchocarpin (LCC) and derricin (DRC), two chalcones isolated from the hexane fraction of roots from Lonchocarpus sericeus. (Poir.) Kunth (Fabaceae), on human platelet aggregation induced by a variety of agonists. LCC and DRC (200 and 400 µg/ml) significantly inhibited in a dose-dependent manner adenosine 5′-diphosphate (ADP)-, arachidonic acid (AA)-, thrombin (THR)-, collagen (COL)-, and adrenalin (ADR)-induced aggregation. Neither LCC nor DRC had their effects potentiated after association with L-arginine (L-ARG), a NO donor, when the inducer was ADP. In contrast, the addition of LCC or DRC to pentoxifylline (PTX), a known phosphodiesterase inhibitor, caused a significant potentiation of platelet inhibition (41.1% and 47.4%) when compared with LCC (20.3%) or DRC (17.9%) alone. The addition of aspirin or yohimbine (YOH) to LCC or DRC did not change their effects on platelet aggregation induced by AA or ADR, respectively. These results suggest that the antiplatelet effect of LCC and DRC may be mediated mainly by phosphodiesterase activity inhibition or elevation of adenosine 3′:5′-cyclic monophosphate (cAMP) and guanosine 3′:5′-cyclic monophosphate (cGMP) intracellular levels or even by inhibition of thromboxane (TX) formation, as these two substances inhibited the aggregation induced by AA, COL, and THR.
Introduction
Platelets and platelet-derived vasoactive agents are important physiological regulators of vascular tone and hemostasis. Evidence indicates that platelet activation plays an important role in the initiation and maintenance of atherosclerosis and thrombotic complications (Ross, Citation1990; Fuster et al., Citation1991). The process of activation is the result of a complex signal transduction cascade brought about by diverse stimulants. This process is regulated in part by levels of the second messengers cAMP and cGMP. Increased intracellular cAMP or cGMP levels lead to inhibition of agonist-induced platelet activation-aggregation and adhesion as well as release of granule contents (Radomski et al., Citation1987). Inhibition of platelet function may be a promising approach to prevent and to treat diseases in which a pathophysiological participation of activated platelets appears likely.
The species Lonchocarpus sericeus. (Poir.) Kunth (Fabaceae), popularly known as ‘angelim.’, as well as other ones (L. araripensis. Benth, L. campestris. Mart), are plants that commonly grow in northeastern Brazil. The presence of pyrrolidine alkaloids (Elbein et al., Citation1984), rotenone (Fang & Casida, Citation1999), and flavonoids (Pereira et al., Citation2000) including chalcones (Gonçalves de Lima et al., Citation1975; Lupi et al., Citation1977) has been described in various species that belong to the Lonchocarpus. genus.
NMR analysis of the hexane fraction from roots of L. sericeus. (HFLS) revealed two major flavonoid constituents: lonchocarpin and derricin. Among flavonoids, chalcones have aroused considerable interest because of their broad pharmacological activity, and reports indicated they possess anti-inflammatory (Hsieh et al., Citation2000), antioxidant (Herencia et al., Citation2001), cytotoxic (El-Subbagh et al., Citation2000), antitumor (Hayashi et al., Citation2000), and antimicrobial activities (Harborne & Williams, Citation2000).
In previous work, we demonstrated the analgesic effect of the hexane fraction from roots of L. sericeus. (Fontenele et al., Citation2001) and the cytotoxic activity of its chalcones (Cunha et al., Citation2003). Despite the knowledge of antiaggregatory activity presented by many flavonoids, including chalcones (Tzeng et al., Citation1991; Sousa et al., Citation1994; Lin et al., Citation1997), there are no data in the literature on the antiplatelet effect of L. sericeus. constituents. Thus, in the current work, we evaluated the antiplatelet activity of derricin and lonchocarpin, two chalcones isolated from this hexane fraction of L. sericeus., in an attempt to elucidate their mechanism of action.
Materials and Methods
Plant material
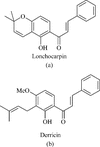
The roots and aerial parts (branchs with leaves and fruits) of Lonchocarpus sericeus. (Poir.) Kunth were collected in Caucaia County, Ceará State, Brazil. A voucher specimen (no. 23171 EAC) identified by Dr. Edson de Paula Nunes (Botanist, Department of Biology, Federal University of Ceará) has been deposited at the Prisco Bezerra Herbarium (Department of Biology, Federal University of Ceará). Root bark and heartwood were separated, air-dried at room temperature, and ground, and chemical constituents were isolated according to the method previously described in Cunha et al. (Citation2003). Briefly, the root bark (2.8 kg) was macerated with hexane for 2 days (repeated twice), to yield 57.3 g of an orange waxy material hexane fraction from roots of L. sericeus. (HFLS) after solvent evaporation. 1H and 13C NMR analysis of the crude extract revealed its composition to be mainly 1:1 mixture of two prenylated flavonoids. HFLS (10.5 g) was adsorbed onto silica gel (10.0 g) and coarsely chromatographed over a layer of silica (30.0 g) in a 5-cm glass column. A fraction of 8.6 g was obtained after exaustive elution with hexane. Successive chromatographies over silica gel of the latter allowed the isolation of pure lonchocarpin (1.3 g) [2″,2″-dimethyldehydropyran(5″,6″:3′,4′)-2′-hydroxychalcone], as orange needles, and pure derricin (0.4 g) [3′-(3,3-dimethylallyl)-2′-hydroxy-4′-methoxychalcone], as an amorphous bright-yellow material. The structures shown in were suggested by extensive NMR analysis, including both one- and two-dimensional techniques (COSY, HMBC, and HMBQ).
Lonchocarpin and derricin were suspended in Cremophor EL (maximum of 2%), and tested for antiplatelet activity. The vehicle was used as control.
Platelet aggregation test
Preparation of platelet-rich and -poor plasma
Blood from healthy volunteers (with previous consent) who had not taken any drug for at least 15 days was collected by venipuncture in a siliconized glass flask containing 3.8% sodium citrate (9:1 v/v). Platelet-rich plasma (PRP) was prepared by centrifugation of blood at 1000 rpm for 7 min at room temperature. Immediately after, platelet-poor plasma (PPP) was obtained by centrifugation of an aliquot of PRP at 3000 rpm for 15 min. The platelets were counted according to the method of Brecher and Cronkite (Citation1950) and adjusted to a concentration of 300,000/mm3 using PPP to dilute PRP.
Aggregation in human platelet-rich plasma
Platelet aggregation was measured using an aggregometer (Chrono-Log Co. Havertown, PA, U.S.A., Model 450), according to the method of Born and Cross (Citation1963). Briefly, platelet aggregation was induced at 37°C in the aggregometer, with stirring at 1000 rpm, by addition of ADP (20 µM), arachidonic acid (AA) (30 µM), thrombin (THR) (0.16 U/mL), collagen (COL) (42.6 microg/ml), or adrenaline (ADR) (30 µM) as agonists. The resulting aggregation, measured as the change in light transmission, was recorded for 8 min and presented as percent aggregation related to control (100%).
Drugs
Adenosine 5′-diphosphate, arachidonic acid, adrenaline, acetylsalicylic acid (aspirin), collagen, L-arginine, and yohimbine were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Bovine thrombin was from Roche (Riode Janeiro, Brazil) and pentoxifylline was from Hoechst (Sao Paulo, Brazil). All other reagents were of analytical grade.
Statistical analysis
All values are expressed as mean±SEM. Differences between the sample-treated and control groups were submitted to analysis of variance (ANOVA) followed by Student-Newman-Keuls test for multiple comparisons (p < 0.05 was considered significant).
Results
Lonchocarpin (LCC) and derricin (DRC) (200 and 400 µg/mL) inhibited the aggregation induced by ADP (20 µM), AA (30 µM), THR (0.16 U/mL), COL (42.6 µg/mL) or ADR (30 µM) in human PRP ().
Table 1.. Effect of LCC and DRC on adenosine 5′-diphosphate (ADP)-, adrenaline (ADR)-, thrombin (THR)-, collagen (COL)- and arachidonic acid (AA)-induced platelet aggregation in human PRP.
L-Arginine is able to influence the response of human platelets stimulated with ADP or COL through a NO-dependent synthesis of cGMP (Anfossi et al., Citation1999). Then, in order to verify whether NO system plays a role in the inhibitory effects of LCC or DRC on ADP-induced aggregation, a positive interplay on platelet response between L-arginine and LCC or DRC was investigated. The results showed that inhibitions of platelet aggregation caused by LCC (22.5%) or DRC (24.5%) alone were not potentiated in the presence of L-arginine (27.9% and 39.8% inhibition, respectively) (), suggesting that platelet-derived NO does not play a significant role in the antiplatelet activity of LCC or DRC. In contrast, the association of LCC or DRC with pentoxifylline, a known phosphodiesterase inhibitor, caused a significant potentiation of the inhibitory effects of both LCC (from 20.3% to 41.1% inhibition) and DRC (from 17.8% to 47.4% inhibition) (). Inhibition of phosphodiesterase activity increases intracelullar cAMP or cGMP levels and regulates the function of platelets (Maurice & Haslam, Citation1990).
Table 2.. Effect of preincubation with L-arginine on antiaggregating actions of LCC or DRC.
Table 3.. Effect of preincubation with pentoxifylline on antiaggregating actions of LCC or DRC.
Aspirin is an antithrombotic drug widely used for prophylaxis or prevention of recurrence of thrombosis and is considered to be effective in some cases of stroke and ischemia. This drug produces antiplatelet effects by inhibiting cyclooxygenase activity, with consequent decreasing in thromboxane A and PGF-prostaglandin F and PGI-prostacyclin (TXA2/PGF2α) formation (Kang et al., Citation1999), but at the same time it also affects blood vessels and decreases the production of PGI2, a biological substance that inhibits the formation of thrombi in blood vessels (Moncada et al., Citation1977). The addition of aspirin to LCC or DRC did not result in potentiation of their effects in the AA-induced platelet aggregation (26.6% and 29.6% inhibition) when compared with LCC and DRC alone (26.4% and 26.4% inhibition, respectively) (). These results suggest that neither LCC nor DRC showed antiplatelet action via the inhibition of cyclooxygenase activity.
Table 4.. Effect of preincubation with aspirin on antiaggregating actions of LCC or DRC.
There are near 300 α2-adrenoceptors on the surface of each human platelet. Through binding to these receptors, adrenaline activates platelets and induces their aggregation subsequent to fibrinogen binding to glycoprotein (GP) IIb/IIIa (Mustonen et al., Citation2000). Owing to the fact that α-adrenergic receptors of human platelets are exclusively of α2A-subtype, yohimbine, one of the most selective α2-adrenoceptor antagonists, has generally been used in platelet studies. Therefore, the effects of yohimbine in association with LCC or DRC on ADR-induced platelet aggregation were assessed. The results showed that the inhibitory effects of LCC (17.2%) or DRC (21.3%) were not modified by their association with yohimbine (14.3% and 22.7% inhibiton, respectively) ().
Table 5.. Effect of preincubation with yohimbine on antiaggregating actions of LCC or DRC.
Discussion
In the current study, we demonstrated that lonchocarpin (LCC) and derricin (DRC), two chalcones isolated from the hexane fraction from roots of Lonchocarpus sericeus., inhibited in a dose-dependent manner the platelet aggregation induced by ADP, ADR, THR, COL, and AA in human PRP. The process of platelet activation starts by an interaction of various agonists with their respective receptors on the platelet membrane. When the activation finally results in the exposure and activation of the glycoprotein (GP) IIb/IIIa receptor, a binding with fibrinogen will take place and platelet aggregation will occur. Inhibition of aggregation can be achieved by either inhibition of membrane receptors or by interception of signaling pathways. Although receptor antagonism provides high specificity, the inhibition of platelet signal transduction is more effective.
The discovery of thromboxane A2 (TXA2) and prostacyclin (PGI2) has generated a great deal of interest in these highly potent active metabolites of AA. TXA2 is the major cyclooxygenase product derived from AA in blood platelets. It is an important mediator of the release reaction and aggregation of platelets (Hornby & Skidmore, Citation1982) and is an extremely potent vasoconstrictor and bronchoconstrictor (Moncada & Vane, Citation1978). The platelet aggregation induced by AA, COL, and THR, which is due to TXA2 formation (Hamberg et al., Citation1975), was inhibited by LCC and DRC. However, their effects were not potentiated by aspirin, a NSAID, that blocks cyclooxygenase synthase activity thereby preventing the production of TXA2. These results suggest that LCC and DRC might be inhibiting a step prior to the cyclooxygenase intervention.
Adrenaline is considered a weak platelet agonist, the function of which is mainly to sensitize platelets to other activating agents. However, physiological adrenaline concentrations enhance both von Willebrand factor–mediated high shear–dependent platelet aggregation and platelet-to-platelet interaction upon collagen. Therefore, an important role for adrenaline in regulating thrombus formation under arterial blood flow condition can be presumed (Hjemdahl et al., Citation1994). Similar to COL and THR, ADR also releases AA from platelets, which is then metabolized by the enzymes cyclooxygenase and TX synthase to produce the proaggregating TXA2. This latter agent increases the intracellular calcium concentration, which is critically involved in the exposure of the GP IIb/IIIa receptor, and in the release of contents of dense and/or alpha granules containing ADP, 5-hydroxytryptamine, platelet factor 4, and various growth factors, including platelet-derived growth factor (Herman, Citation1998). In the current work, yohimbine, an α2-adrenoceptor antagonist, was used in association with LCC and DRC when the inducer was ADR. Our results showed that LCC and DRC did not have their effects modified by this association. Therefore, LCC and DRC do not appear to exert their effects on ADR-induced aggregation through an α2-adrenoceptor blockade. In this case, it is possible that their effects are related to the inhibition of TXA2 production.
The release of NO from L-arginine by vascular endothelium provides a powerful mechanism for the inhibition of platelet adhesion and aggregation, causing disaggregation of platelets both in vitro. and in vivo. (Radomski & Moncada, Citation1993). This molecule acts as a potent inhibitor of platelet responses to agonists via activation of intraplatelet soluble guanylyl cyclase, which leads to an increase in the intracellular levels of cGMP. Because L-arginine provides a guanidino nitrogen group for NO synthesis through NO synthase activity, we studied its effect in association with LCC and DRC on human platelet aggregation induced by ADP. The inhibitory effects of LCC and DRC were not modified by the addition of L-arginine. These results suggest that the antiaggregation effects of LCC and DRC are not exerted through the NO pathway.
Cyclic nucleotides are very important in modulating platelet functions. An elevation of cyclic nucleotide levels, either by activation of adenylyl and guanylyl cyclase or by inhibition of phosphodiesterase, is the most potent inhibitory pathway to regulate platelet activation (Liao et al., Citation1998). Both cyclic nucleotides (cAMP and cGMP) are involved in platelet responses, including aggregation, ATP release, protein phosphorylation, intracellular calcium mobilization, and GP IIb/IIIa activation (Herstrup et al., Citation1994).
Pentoxifylline (PTX) is a dimethylxanthine derivative that exerts physiological and pharmacological effects by several mechanisms including translocation of extracellular calcium, increases in cAMP and cGMP caused by inhibition of phosphodiesterases, and blockade of adenosine receptors (Cunha et al., Citation2000). Besides the inhibitory effect on platelet aggregation, PTX also causes platelet disaggregation both in vitro. and in vivo. (Sidorova et al., Citation1991; De La Cruz et al., Citation1993). The association of PTX with LCC or DRC significantly increased the inhibitory effects of these two chalcones in platelet aggregation, suggesting that both substances may exert their actions through the phosphodiesterase inhibition and increase in cAMP and/or cGMP levels. Recently, highly specific inhibitors for each of the phosphodiesterase isoforms have been developed for therapeutic uses. These inhibitors that elevate cAMP and/or cGMP levels of platelets and inhibit platelet aggregation and adhesion have therapeutic possibilities in angina and thrombosis treatments (Ohba et al., Citation2001).
Platelet aggregation may play a pathophysiological role in a variety of thromboembolic disorders, including myocardial infarction, cerebrovascular diseases, and atherosclerosis. Therefore, prevention of platelet aggregation by drugs should provide effective prophylactic and/or therapeutic means of treating such disorders. The current observations that LCC and DRC inhibit platelet aggregation, possibly by mechanisms involving the inhibition of phosphodiesterase activity and increases in cAMP and/or cGMP levels or alternatively by inhibition of TXA2 formation, provides a good rationale for the therapeutic use of these agents alone or in combination with other ones in thromboembolic disorders.
Acknowledgments
This work was financially supported by the Brazilian National Research Council (CNPq). The authors are grateful to Ms. Maria Vilani R. Bastos and Ms. Jacqueline de Almeida Viana for technical assistance.
References
- Anfonssí G, Russo I, Massuco P, Mattiello L, Perna P, Tassone F, Trovati M (1999): L-Arginine modulates aggregation and intracellular cyclic 3′,5′-guanosine monophosphate levels in human platelets: Direct effect and interplay with antioxidative thiol agent. Thromb Res 94: 307–316. [CSA]
- Born GVR, Cross MJ (1963): The aggregation of blood platelet. J Physiol 168: 178–195. [PUBMED], [INFOTRIEVE], [CSA]
- Brecher G, Cronkite EP (1950): Morphology and enumeration of human blood platelets. J Appl Physiol 3: 365–372. [PUBMED], [INFOTRIEVE], [CSA]
- Cunha GMA, Bezerra PJP, Saldanha MDD, Cavalcante MC, De-Bruin VMS, Viana GSB (2000): Pentoxifylline improves learning and memory in glutamate-lesioned rats. Pharmacol Biochem Behav 66: 687–694. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cunha GMA, Fontenele JB, Nobre-Júnior HV, Martins-de-Souza FC, Silveira ER, Nogueira NAP, Moraes MO, Viana GSB, Costa-Lotufo LV (2003): Cytotoxic activity of chalcones isolated from Lonchocarpus sericeus. (Poir.) Kunth. Phytother Res 17: 155–159. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- De la Cruz JP, Romero MM, Sanchez P, Sanchez de la Cuesta F (1993): Antiplatelet effect of pentoxifylline in human whole blood. Gen Pharmacol 24: 605–609. [PUBMED], [INFOTRIEVE], [CSA]
- Elbein AD, Mitchell M, Sanford BA, Fellows LE, Evans SV (1984): The pyrrolidine alkaloid, 2,5-dihydroxymethyl-3,4-dihydroxypyrrolidine, inhibits glycoprotein processing. J Biol Chem 259: 12409–12413. [PUBMED], [INFOTRIEVE], [CSA]
- El-Subbagh HI, Abu-Zaid SM, Mahran MA, Bradia FA, Al-Obaid AM (2000): Synthesis and biological evaluation of certain alpha, beta-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem 43: 2915–2921. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fang N, Casida JE (1999): Cube resin insecticide: Identification and biological activity of 29 rotenoid constituents. J Agric Food Chem 47: 2130–2136. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fontenele JB, Cardoso-De-Assis RK, Silveira ER, Viana GSB (2001): Analgesic effect of hexane fraction from roots of Lonchocarpus sericeus.. Pharm Biol 39: 429–434. [CSA]
- Fuster V, Badimon JJ, Chesebro JH (1991): The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 326: 310–318. [CSA]
- Gonçalves de Lima O, De Méllo JF, De Barros Coêlho JS, De Andrade Lyra FD, Machado de Albuquerque M, Marini-Bettólo GB, Delle Monache G, Delle Monache F (1975): New prenylated chalcones from Lonchocarpus neuroscapha. Benth. (Cordoa piaca.). Farmaco [Sci] 30: 326–342. [CSA]
- Hamberg M, Svenson J, Samuelsson B (1975): Thromboxane: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA 72: 2994–2995. [PUBMED], [INFOTRIEVE], [CSA]
- Harborne JB, Williams CA (2000): Advances in flavonoid research since 1992. Phytochemistry 55: 481–504. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hayashi A, Gillen AC, Lott JR (2000): Effects of daily oral administration of quercetin chalcone and modified citrus pectin. Altern Med Rev 5: 546–552. [PUBMED], [INFOTRIEVE], [CSA]
- Herencia F, Ferrandiz ML, Ubeda A, Guillen I, Dominguez JN, Charris JE, Lobo GM, Lebeau J, Furman C, Bernier J, Duriez P, Teissier E, Cotelle N (2001): Antioxidant properties of di-tert.-butylhydroxylated flavonoids. Free Radic Biol Med 29: 900–912. [CSA]
- Herman, AG (1998): Rationale for the combination of anti-aggregating drugs. Thromb Res 92: S17–S21. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Herstrup K, Jablonka B, Honig-Liedl P, Just M, Kochsiek K (1994): Phosphorylation of focal adhesion vasodilator-stimulated phosphoprotein at Ser 157 in human platelets correlates with fibrinogen receptor inhibition. Eur J Biochem 225: 21–27. [CSA], [CROSSREF]
- Hjemdahl P, Chronos NAF, Wilson DJ, Bouyloux P, Goodall AH (1994): Epinephrine sensitizes human platelets in vivo. and in vitro. as studied by fibrinogen binding and p.-selectin expression. Arterioscler Thromb 14: 77–84. [PUBMED], [INFOTRIEVE], [CSA]
- Hornby EJ, Skidmore IF (1982): Evidence that prostaglandin endoperoxides can induce platelet aggregation in the absence of thromboxane A2 production. Biochem Pharmacol 31: 1158–1160. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hsieh HK, Tsao LT, Wang JP, Lin CN (2000): Synthesis and anti-inflammatory effect of chalcones. J Pharm Pharmacol 52: 163–171. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kang WS, Lim IH, Yuk DY, Chung KH, Park JB, Yoo HS, Yun YP (1999): Antithrombotic activities of green tea catechins and (-)-epigallocatechin gallate. Thromb Res 96: 229–237. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Liao CH, Tzeng CC, Teng CM (1998): Cyclic AMP and cyclic GMP phosphodiesterase inhibition by an antiplatelet agent 6-[(3-methylene-2-oxo-5-phenyl-5-tetrahydrofuranyl) methoxy] quinolinone (CCT-62). Eur J pharmacol 349: 107–114. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lin CN, Lee TH, Hsu MF, Wang JP, Ko FN, Teng CM (1997): 2′,5′-Dihydroxychalcone as a potent chemical mediator and cyclooxygenase inhibitor. J Pharm Pharmacol 49: 530–536. [PUBMED], [INFOTRIEVE], [CSA]
- Lupi A, Delle Monache G, Delle Monache F, Marini-Bettólo GB, Gonçalves de Lima O, De Mello JF (1977): Synthetic analogs of natural prenylated and chromene chalcones. Farmaco [Sci] 32: 261–269. [CSA]
- Maurice DH, Haslam RJ (1990): Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: Inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol 37: 671–681. [PUBMED], [INFOTRIEVE], [CSA]
- Moncada S, Higgs EA, Vane JR (1977): Human arterial and venous tissues generate prostacyclin (prostaglandin X), a potent inhibitor of platelet aggregation. Lancet 309: 18–20. [CSA], [CROSSREF]
- Moncada S, Vane JR (1978): Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2 and prostacyclin. Pharmacol Rev 30: 293–331. [PUBMED], [INFOTRIEVE], [CSA]
- Mustonen P, Savola J-M, Lassila R (2000): Atipamezole, an imidazoline-type α2-adrenoceptor inhibitor, binds to human platelets and inhibits their adrenaline-induced aggregation more effectively than yohimbine. Thromb Res 99: 231–237. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ohba Y, Soda K, Zaitsu K (2001): A sensitive assay of human blood platelet cyclic nucleotide phosphodiesterase activity by HPLC using fluorescence derivatization and its application to assessment of cyclic nucleotide phosphodiesterase inhibitors. Biol Pharm Bull 24: 567–569. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pereira AS, Afonso Serrano MA, Aquino Neto FR, Cunha Pinto A, Furtado Teixeira D, Gilbert B (2000): Analysis and quantification of rotenoids and flavonoids in Derris (Lonchocarpus urucu.) by high-temperature high-resolution gas chromatography. J Chromatogr Sci 38: 174–180. [PUBMED], [INFOTRIEVE], [CSA]
- Radomski MW, Moncada S (1993): The biological and pharmacological role of nitric oxide in platelet function. Adv Exp Med Biol 344: 251–264. [PUBMED], [INFOTRIEVE], [CSA]
- Radomski MW, Palmer RM, Moncada S (1987): Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 7: 1057–1058. [CSA], [CROSSREF]
- Ross R (1990): The pathogenesis of atherosclerosis: A perspective for the 1990's. Nature 362: 801–809. [CSA], [CROSSREF]
- Sidorova LD, Domnikova NP, Logvinenko AS (1991): [Platelet aggregation inhibitors in the comprehensive treatment of bronchial asthma]. Klin Med 69: 47–49. [CSA]
- Sousa MF, Cunha GMA, Fontenele JB, Viana GSB, Rao VSN (1994): Antithrombotic activity of ternatin, a tetrametoxy flavone from Egletes Viscosa Less.. Phytother Res 8: 478–481. [CSA]
- Tzeng SH, Ko WC, Ko FN, Teng CM (1991): Inhibition of platelet aggregation by some flavonoids. Thromb Res 64: 91–100. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]