Abstract
The current study investigated the putative antidepressant-like effect of paeonol, a phenolic component from the root bark of Paeonia suffruticosa. Andr. (Ranunculaceae), in mice or rats using the tail suspension test (TST) and forced swimming test (FST), two widely accepted pharmacological models for detecting antidepressant activity in modern medicinal researchers. It was found that paeonol, at doses of 20, 40, and 80 mg/kg (p.o.), reduced immobility time in TST and FST in mice. Paeonol also decreased immobility time at 25 and 50 mg/kg (p.o.) in the FST in rats. Furthermore, paeonol as well as fluoxetine (FLU), at the doses tested, did not produce significant effects on locomotor activity. With 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and lactic dehydrogenase (LDH) assay, 5, 10, and 20 µM paeonol or a classical antidepressant, 10 µM FLU, protected PC12 cells from the lesion induced by 10 µM corticosterone (Cort) treatment for 48 h. In the fura-2/AM (acetoxymethyl ester) labeling assay, 10 and 20 µM paeonol, 10 µM FLU attenuated the intracellular Ca2+ overloading induced by 200 µM Cort treatment for 48 h in PC12 cells. In summary, we first demonstrated that paeonol possessed an antidepressant-like action through the protection of PC12 cells as well as reversal intracellular Ca2+ overloading.
Introduction
Depression is a major disease affecting nearly 13–20% of the population (Licinio & Wong, Citation1999). Although many novel antidepressants emerge and possess certain therapeutic actions, most of them have inevitably some serious adverse effects. Accordingly, there is an urgent need for the research and development of more effective antidepressants without any (or with lower) adverse effect. In Oriental society, herbs and herbal preparations are widely used by consumers and have a long history of use as medicines. Some of them may be effective alternatives in the treatment of depression. Paeonol (), a major phenolic component of Moutan Cortex, the root bark of Paeonia suffruticosa. Andr. (Ranunculaceae), is described to have anxiolytic (Mi et al., Citation2005), antidysrhythmic, antiatherosclerotic, and anticancer activities (Lv & Liu, Citation2005). Previous report suggested that paeonol inhibited monoamine oxidase A and B (MAO-A and MAO-B) in a dose-dependent manner (Kong et al., Citation2004), which plays a central role in several psychiatric and age-related neurological disorders, including clinical depression and Parkinson disease (Mazzio et al., Citation1998).
The pathogenesis of depression, as well as the underlying mechanisms of action of currently available antidepressant treatments, is as yet unclear (Wong & Licinio, Citation2001). It is known that the hypothalamic-pituitary-adrenal (HPA) axis is hyperactive in depressive patients, even hypercorticism has occurred, which may be caused by the adaptive failure of HPA axis activity (Takeda et al., Citation2000; Mizoguchi et al., Citation2001). The glucocorticoids (GCs) receptors in hippocampus are the richest in rat brain. The GCs receptors are downregulated in depressive patients because of the high levels of GCs, and the negative feedback of hippocampus on the HPA axis is attenuated consequently, thereby, the high blood levels of GCs are sustained permanently (Takeda et al., Citation2000; Mizoguchi et al., Citation2001). Because the hippocampus is a major site for emotional processes, a role for excessive long-lasting plasma GCs levels has been suggested in conditions of mental impairment. The occurrence of depression may be closely related to the GCs-induced lesions in the hippocampus (Sapolsky, Citation2000a). It is therefore important to find pharmacological strategies that will avert or reduce these potential consequences on brain function. The rat adrenal pheochromocytoma cell line, designated PC12 cells, possesses typical features of brain neurons and is abundant with GCs receptors (Anderson & Michelsohn, Citation1989). In this study, the antidepressant effect of paeonol was evaluated in three depressive animal models (Porsolt et al., Citation1978; Muscat et al., Citation1990); high concentration of corticosterone was incubated with PC12 cells to simulate the lesion state of brain neurons in depressive illness to observe the neuroprotective action of paeonol.
Materials and Methods
Reagents and chemicals
Paeonol was purchased from Xuanchengbaicao Botanic Department (Anhui, China). Corticosterone and fluoxetine (FLU; purity 99%) were bought from Sigma (St. Louis, MO, USA). DMEM culture medium was from Gibco BRL (Grand Island, NE, USA); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Merck Chemical Co. (Shanghai, China); Fura-2/AM was obtained from Fluka (Stockholm, Sweden). Fluoxetine and paeonol were both ultrasonically dispersed in distilled water to which Tween-80 (4 drops/10 ml) had been added.
Animals
Male KM mice weighing 18–22 g and male Wistar rats weighing 220–260 g were used. For the TST and FST, mice were randomly assigned to six equal groups (vehicle control, 10 mg/kg fluoxetine or 10, 20, 40, or 80 mg/kg paeonol). For the FST, rats were randomly assigned to five experimental groups (vehicle control, 10 mg/kg fluoxetine or 12.5, 25, or 50 mg/kg paeonol). Each experimental group consisted of ten animals. The animals were housed under controlled conditions of light (12-h light/dark cycle, lights on at 7:00 a.m.) and temperature (25 ± 1°C) with free access to food and water. All the animals were purchased from Experimental Animal Centre of China Pharmaceutical University. Experiments followed a protocol approved by the local animal ethics committee and the local government. All experiments were in accordance with the recommendations of the European Union regarding animal experimentation (Directive of the European Council 86/609/EC).
Tail suspension test (TST)
Mice were given paeonol (10, 20, 40, 80 mg/kg, p.o.) or distilled water once daily for 7 days. The test was performed 1 h after the last administration. FLU (10 mg/kgi.p.) was administered only once, 1 h prior to the test. The mice were suspended by the tail to the edge of a shelf 75 cm above the floor. The tail was secured to the shelf by adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility was recorded only during the last 4 min of the total 6-min test period. Mice were considered immobile only when they hung passively and completely motionless.
Forced swimming test (FST)
Mice were placed in individual glass cylinders (20 cm height × 10 cm diameter) containing 10 cm of water at 22–25°C for 6 min. The duration of immobility was scored during the last 4 min of the 6-min test period. A mouse was recorded as immobile when floating motionless or making only those movements necessary to keep its head above water. Mice were given paeonol (10, 20, 40, or 80 mg/kg, p.o.) or distilled water once daily for 7 days. The last dose was given 1 h before the test. FLU (10 mg/kg i.p.) was administered only once 1 h prior to testing. In a variant of this procedure for rat experiments, the animals were placed in clear glass cylinders (40 cm height × 18 cm diameter) filled with water (22–25°C) to a depth of 23 cm for 15 min. The rats were then dried and returned to their home cages. Almost all rats had immobility time of more than 90 s during the last 5 min in the training session, and the animals that passed the criterion were used in the next test session. Before the test session on the following day, the rats were given paeonol (12.5, 25, 50 mg/kg, p.o.) or distilled water for 7 successive days. FLU (10 mg/kg i.p.) was administered only once, 1 h before testing. In the test session, the rats were exposed to the cylinders as described above.
Measuring locomotor activity
In order to detect any association of immobility in the FST with changes in motor activity, the activities of animals treated with the paeonol were tested in an open field. Mice were randomly assigned to five equal groups (vehicle control, 10 mg/kg fluoxetine or 20, 40, or 80 mg/kg paeonol), and each experimental group consisted of ten mice. Each mouse was placed in the center of the open field apparatus, and the locomotor activity was assessed immediately before the FST. The open field apparatus was a field, 70 cm in diameter, which was demarcated into 18 approximately equal areas. Hand-operated counters were used to score locomotion (number of line crossings within 5 min) and rearing frequencies (number of times an animal stood on its hind legs). A separate researcher, who was blind to the treatment group, scored the behavior in the open field. The openfield apparatus was washed with a deodorant solution (0.5% ammonia, 15% ethanol, 10% extran, 5% isopropanol, 10% pinol, and 59.5% water) before each behavioral test to eliminate possible odor clues left by previous subjects. Experiments were performed in a dark room, and the apparatus was illuminated by a 60-W bulb positioned 1 m above the center of the circle (Sun et al., 2005).
PC12 cells culture and evaluation of cell viability
The pheochromocytoma cells (PC12) were purchased from Shanghai Institute of Cell Biology and Biochemistry, Chinese Academy of Sciences. The cells were seeded into 96-well plates at a density of 2 × 105/ml and cultured in the medium consisting of 90% Dulbecco's modified Eagle medium (DMEM), 5% heat-inactivated horse serum, 5% fetal calf serum, 200 kU/l benzylpenicillin, and 100 mg/l streptomycin in a humidified incubator with 5% CO2, 37°C for 3–4 days. The cells were incubated with serum-free low-glucose DMEM containing 10 µM Cort and paeonol (5, 10, and 20 µM), fluoxetine (FLU) (10 µM). The medium was collected and LDH activity, which was quantified as an index of cell death, was determined with a LDH assay kit. The reaction mixture was monitored at 340 nm with a spectrophotometer (Vital Scientific, Dieren, The Netherlands) and the LDH activity was calculated from the decrease of NADH absorbance resulting from the conversion of pyruvate to lactate. In the MTT assay, after two washes with D-Hanks, the cells were incubated with DMEM containing 0.5 mg/ml MTT for another 4 h at 37°C. A total of 100 µl dimethyl sulfoxide (DMSO) was subsequently added to each well to dissolve formazan crystals. Absorbance at 490 nm (A490nm values) was detected with a Versamax spectrophotometer (Molecular Devices, Sunyvale, CA, USA).
Measurement of intracellular Ca2+ concentration
The [Ca2+]i in PC12 cells was monitored by fluorometry, using the Ca2+-sensitive dye, Fura-2/AM. The cells were seeded in a 24-well plate at the density 2 × 105/ml and cultures for 3–4 days. The medium was replaced with serum-free DMEM containing paeonol (5, 10, and 20 µM), FLU (10 µM) in the presence of 200 µM Cort, and the cells were then cultured for another 24 h. For Fura-2/AM loading, cells were collected and incubated with the complete medium containing 5 µM Fura-2/AM at 37°C for 45 min. Subsequently, the cells were washed and resuspended again with cold balanced salt solution (BSS) buffer (130 mM NaCl, l5.4 mM KCl, 1.8 mM CaCl2, 5.5 mM glucose, 20 mM HEPES, pH 7.4) containing 0.2% bovine serum albumin. The cells were incubated at 37°C for another 5 min just prior to measurement. [Ca2+]i was determined by alternating excitation wavelengths of alternating excitation wavelength of between 340 and 380 nm with emission at 510 nm, using a fluorescence spectrophotometer (F-4500, HITACHI, Japan) and the data were analyzed with customized software provided by F-4500. The ratio of fluorescence intensities excited by 340 or 380 nm was calculated after subtraction of the background fluorescence.
Statistical analysis
The raw data were analyzed statistically. All the results are expressed as the means ± SEM. Non-parametric tests were used for all statistical comparisons. Overall differences according to the treatment were confirmed using the Student's t.-test. Statistical differences were considered significant at p < 0.05.
Results
Antidepressant-like effects of paeonol in mice
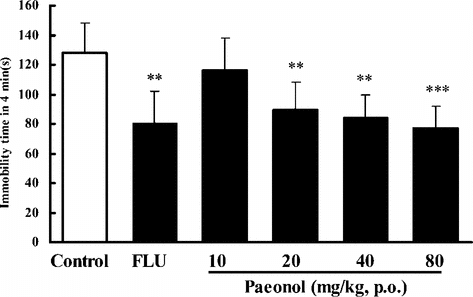
In the TST, pretreatment of the mice once daily for 7 days with paeonol at doses of 20, 40, and 80 mg/kg (p.o.) produced significant reductions in the duration of immobility, by 25%, 29%, or 35%, respectively (). Treatment with paeonol at the dose of 20, 40, or 80 mg/kg (p.o.) for 7 days also reduced the immobility time by 30%, 35%, or 40%, in the FST in mice (). FLU (10 mg/kg i.p.) reduced the immobility time by 30–37% in these experiments.
Figure 2 Effect of paeonol on immobility time in the tail suspension test in mice. In paeonol groups, the mice were treated with distilled water or paeonol (p.o.) once a day for 7 days. The test was performed 1 h after the last administration of paeonol. In the positive control, fluoxetine (FLU) was given only once (i.p.) 1 h prior to the test. Data are expressed as means ± SEM. **p < 0.01, ***p < 0.001 versus control.
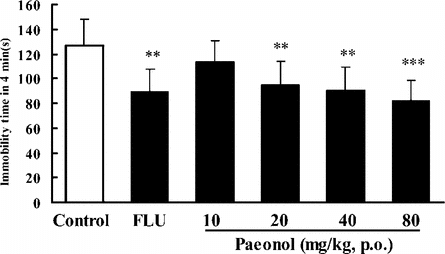
Figure 3 Effects of paeonol on immobility time in the forced swimming test in mice. In paeonol groups, mice were treated with distilled water or paeonol (p.o.) once a day for 7 days. The tests were performed 1 h after the last administration of paeonol. In the positive control, fluoxetine (FLU) was given only once (i.p.) 1 h prior to the test. Data are expressed as means ± SEM. **p < 0.01, ***p < 0.001 versus corresponding control.
Antidepressant-like effects of paeonol in rats
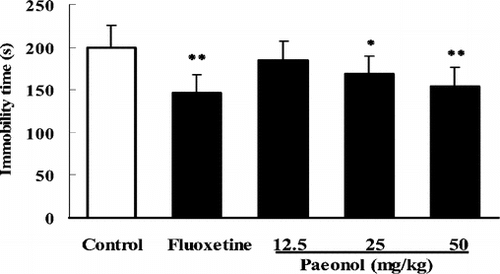
After 25 or 50 mg/kg (p.o., ) of paeonol was administered, the immobility time in the FST in rats was also significantly reduced by 17% or 22% (p.o.). FLU (10 mg/kg i.p.) reduced the immobility time by 26% in this test
Figure 4 Effects of paeonol on immobility time in the forced swimming test in rats. In paeonol groups, the rats were treated with paeonol (p.o.) prior to the tests. In the positive control, fluoxetine (FLU) was given only once (i.p.) 1 h before the tests. Data are expressed as means ± SEM. *p < 0.05, **p < 0.01 versus corresponding control.
Effect of paeonol on the spontaneous motor activity in mice
In order to determine whether paeonol really has an antidepressant-like action, we have to find whether paeonol has excitatory or inhibitory actions on the central nervous system. The 20–80 mg/kg (p.o.) paeonol had no effect on spontaneous motor activity in mice as shown in , indicating that paeonol had no excitatory or inhibitory action in the central nervous system at least at the doses of 20–80 mg/kg (p.o.). All these results indicate that paeonol has an antidepressant-like effect in mice and rats.
Table 1 Effects of 7-day treatments with paeonol and FLU on the open field parameters recorded for 5 min in mice.
Protective effects of paeonol on PC12 cells from the lesion induced by corticosterone
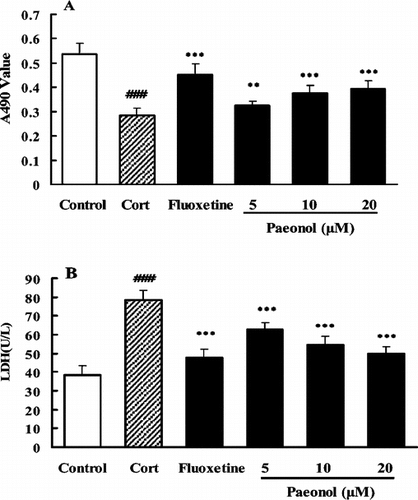
After the treatment of PC12 with Cort 10 µM for 48 h, the A490nm values decreased and LDH activity increased markedly compared with control (p < 0.001), indicating that the cells were impaired or some of them were dead. FLU (10 µM) or paeonol (5, 10, and 20 µM) reversed the changes of cell viability. Compared with their effects on the corresponding Cort treatment group, the drugs as mentioned above increased the A490nm values and decreased LDH release significantly, indicating that paeonol or FLU could protect the cells from the lesion induced by Cort ().
Figure 5 Protective effect of paeonol on the PC12 cells from the lesion induced by corticosterone. Cells were exposed to corticosterone 10 µM in the absence or presence of paeonol or fluoxetine for 48 h; cell viability was measured using a colorimetric MTT assay (A) and LDH assay (B). Data are expressed as means ± SEM. ###p < 0.001 versus corresponding control. **p < 0.01 and ***p < 0.001 versus corresponding corticosterone-treated groups.
Effects of paeonol on corticosterone-induced [Ca2+]i overloading in PC12 cells
After the treatment of PC12 cells with Cort 200 µM for 48 h, [Ca2+]i elevated significantly compared with the control (p < 0.001). While in the presence of paeonol (10 and 20 µM) or FLU (10 µM), the Cort-induced [Ca2+]i overloading was attenuated (). These results indicated that the cytoprotective action of antidepressants might be associated with their reducing the [Ca2+]ioverloading.
Figure 6 Effect of paeonol or fluoxetine on the corticosterone-induced [Ca2+]i overloading in PC12 cells. Cells were exposed to corticosterone 200 µM in the absence or presence of paeonol or fluoxetine for 48 h, and [Ca2+]i was detected using the sensitive indicator dye, Fura-2/AM. Data are expressed as means ± SEM. ###p < 0.001 versus control. **p < 0.01, ***p < 0.001 versus corticosterone-treated group.
![Figure 6 Effect of paeonol or fluoxetine on the corticosterone-induced [Ca2+]i overloading in PC12 cells. Cells were exposed to corticosterone 200 µM in the absence or presence of paeonol or fluoxetine for 48 h, and [Ca2+]i was detected using the sensitive indicator dye, Fura-2/AM. Data are expressed as means ± SEM. ###p < 0.001 versus control. **p < 0.01, ***p < 0.001 versus corticosterone-treated group.](/cms/asset/38769c31-45ff-44f8-8ffa-36cbb6251d4c/iphb_a_168527_f0006_b.gif)
Discussion
The tail suspension and forced swimming tests are two behavioral tests in rodents that predict the clinical efficacy of many types of antidepressant treatments (Porsolt et al., Citation1977Citation1978; Butterweck et al., Citation1998). The tests are sensitive and specific to all major classes of antidepressant drugs including tricyclics, serotonin specific reuptake inhibitors, and monoamine oxidase inhibitors (Borsini et al., Citation1988; Detke et al., Citation1995). We studied paeonol on the immobility behaviors in TST and FST in mice and rats. The oral doses of paeonol given to animals in our tests were referred to the anxiolytic effect of paeonol (Mi et al., Citation2005). In that study, paeonol (at an oral dose of 8.75, 17.5, 35, 70, or 140 mg/kg) induced no significant increase in the number of squares entered in open field test. In the present study, we investigated the anti-immobility activity of paeonol at 10, 20, 40, or 80 mg/kg in mice and 12.5, 25, or 50 mg/kg in rats in TST or FST. The results demonstrate that paeonol at oral doses of 20, 40, or 80 mg/kg in the TST and the FST in mice and 25 or 50 mg/kg in the FST in rats for 7 days significantly decreased the duration of immobility. As changes in immobility may be due to changes in locomotor activity caused by central nervous system stimulating agents, mice were tested in the open filed test. Neither paeonol nor fluoxetine, at the doses tested, produced significant effects on locomotor activity. These data show that paeonol has antidepressant effects in animal models of immobility tests.
It was reported that volumes of the double-side hippocampus were reduced in patients with major depression compared with healthy controls, and there was a positive correlation between hippocampus atrophy and the time course of the depression (Sapolsky, Citation2000bCitationc). Chronic psychosocial stress caused apical dendritic atrophy of hippocampal CA3 pyramidal neurons, which may be mediated by activation of the hypothalamic-pituitary-adrenal (HPA) axis acting in concert with the endogenous excitatory amino acid release (Magarinos et al., Citation1996; Sapolsky, Citation2000bCitationc). Mizoguchi and colleagues (Citation2002Citation2003) postulated that preventing the attenuation of glucocorticoid feedback is one of the major mechanisms of its antidepressant activity. Mizoguchi et al. (Yokoyama et al., Citation1981)suggested that glucocorticoid secreted by saikosaponins enhanced the feedback mechanism vulnerable to stress (Yokoyama et al., Citation1981). Ayensu and co-workers (Citation1995) reported high glucocorticoid levels after chronic mild stress exposure in rats, while other authors have not found elevated levels of this hormone after prolonged stress situations (Azpiroz et al., Citation1999). Moreover, Rittenhouse and colleagues (Citation2002) suggested that the increased behavioral immobility and exaggerated secretion of stress hormones by Wistar Kyoko rats during swimming stress was partly explained by the reduced sensitivity of glucocorticoid receptors that supplied negative feedback to the HPA axis.
To investigate the possible mechanism of action underlying the antidepressant-like activity of paeonol deeply, we then incubated PC12 cells with Cort to observe the cytoprotection of paeonol. After the treatment of PC12 with Cort 10 µM for 48 h, the A490nm values decreased and LDH activity increased markedly compared with control, while paeonol (5, 10, and 20 µM) reversed the changes of cell viability. Compared with its effect on the corresponding Cort treatment group, paeonol increased the A490nm values and decreased LDH release significantly, indicating that paeonol could protect the cells from the lesions induced by Cort.
There is evidence suggesting that three kinds of classical antidepressants all can protect the primary cultured rat hippocampal neurons, as well as PC12 cells from the lesions induced by Cort, while antipsychotic drug chlorpromazine or anxiolytic diazepam have no such effect (Li et al., Citation2003). In more detail, down-regulation of [Ca2+]i overloading and up-regulation of NGF mRNA level in the Cort-treated cells may be partly underlying the cytoprotective action of antidepressants (Li et al., Citation2003). The mechanism of Cort-induced lesion of neurons is still unclear, which may be closely related to the glucose metabolism disorder or [Ca2+]i overloading in neurons (De Leon et al., Citation1997; Kim & Yoon, Citation1998). In this study, Cort did induce the [Ca2+]i overloading in PC12 cells, while paeonol or FLU reversed these changes, which also might be one of the mechanisms of its cytoprotection.
Taken together, these results lead us to conclude that paeonol appears to have a certain antidepressant activity in mouse and rat models, and the cytoprotective effect of paeonol may be consistent (at least part) with its antidepressant-like property. Paeonol has no excitatory or inhibitory action on the central nervous system and toxicity. Moreover, it can be taken orally. All of these characteristics provide promise for further studies and development of paeonol for new antidepressants.
Acknowledgment
This research was supported by China Pharmaceutical University, Nanjing, People's Republic of China.
References
- Anderson DJ, Michelsohn A (1989): Role of glucocorticoids in the chromaffin neuron developmental decision. Int J Dev Neurosci 7: 475–487, [INFOTRIEVE], [CSA]
- Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowski DS (1995): Effects of chronic mild stress on serum complement activity, saccharin preference and corticosterone levels in Flinders lines of rats. Physiol Behavior 57: 165–169, [CROSSREF], [CSA]
- Azpiroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF (1999): Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferative response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology 24: 345–361, [INFOTRIEVE], [CROSSREF], [CSA]
- Borsini F, Meli, Alberto (1988): Is the forced swimming test a suitable model for revealing antidepressant activity? Revi Psychopharmacol 94: 147–160, [CSA]
- Butterweck V, Petereit F, Winterhoff H, Nahrstedt A (1998): Solubilized hypericin and pseudohypericin from Hypercum perforatum. exert antidepressant activity in the forced swimming test. Planta Med 64: 291–294, [INFOTRIEVE], [CSA]
- De Leon MJ, McRae T, Rusinek H, Convit A, De Santi S, Tarshish C, Golomb J, Volkow N, Daisley K, Orentreich N, McEwen B (1997): Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer's disease. Clin Endocrinol Metab 82(10): 3251–3259, [CROSSREF], [CSA]
- Detke MJ, Rickels, Michael, Lucki, Irwin (1995): Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121: 66–72, [INFOTRIEVE], [CROSSREF], [CSA]
- Kim JJ, Yoon KS (1998): Stress: Metaplastic effects in the hippocampus. Trends Neurosci 21(12): 505–509, [INFOTRIEVE], [CROSSREF], [CSA]
- Kim SH, Han J, Seog DH, Chung JY, Kim N, Hong Park Y, Lee SK (2005): Antidepressant effect of Chaihu-Shugan-San extract and its constituents in rat models of depression. Life Sci 76: 1297–1306, [INFOTRIEVE], [CROSSREF], [CSA]
- Kong LD, Cheng CH, Tan RX (2004): Inhibition of MAO A and B by some plant-derived alkaloids, phenols and anthraquinones. J Ethnopharmacol 91: 351–355, [INFOTRIEVE], [CROSSREF], [CSA]
- Licinio J, Wong ML (1999): The role of inflammatory mediators in the biology of major depression: Central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatr 4: 317–327, [CROSSREF], [CSA]
- Li YF, Liu YQ, Huang WC, Luo ZP (2003): Cytoprotective effect is one of common action pathways for antidepressants. Acta Pharmacol Sin 24: 996–1000, [INFOTRIEVE], [CSA]
- Lv CM, Liu HY (2005): Advances in pharmacological studies of paeonol. Herald of Medicine 24: 142–143, [CSA]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E (1996): Chronic psychosocial stress caused apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16: 3534–3540, [INFOTRIEVE], [CSA]
- Mazzio EA, Harris N, Soliman KF (1998): Food constituents attenuate oxidase activity and peroxide levels in C6 cells. Planta Med 64: 603–607, [INFOTRIEVE], [CSA]
- Mi, Xiao Juan, Si Wei Chen, Wen Juan Wang, Rui Wang, Yi Jing Zhang, Wei Jing Li, Yu Lei Li (2005): Anxiolytic-like effect of paeonol in mice. Pharmacol Biochem Behav 81: 683–687, [INFOTRIEVE], [CROSSREF], [CSA]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T (2001): Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology 26: 443–459, [INFOTRIEVE], [CROSSREF], [CSA]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Tabira T (2002): Saiko-ka-ryukotsu-borei-to, an herbal medicine, prevents chronic stress-induced disruption of glucocorticoid negative feedback in rats. Life Sci 72: 67–77, [INFOTRIEVE], [CROSSREF], [CSA]
- Mizoguchi K, Yuzurihara M, Ishige A, Aburada M, Tabira T (2003): Saiko-ka-ryukotsu-borei-to, a herbal medicine, ameliorates chronic stress-induced depressive state in rotarod performance. Pharmacol Biochem Behav 75: 419–425, [INFOTRIEVE], [CROSSREF], [CSA]
- Muscat R, Sampson D, Willner P (1990): Dopaminergic mechanism of imipramine action in an animal model of depression. Biol Psychiaty 28: 223–230, [CROSSREF], [CSA]
- Porsolt RD, Le Pichon M, Jalfre M, Chatterjee SS (1977): Depression: A new animal model sensitive to antidepressant treatments. Nature 266: 730–732, [INFOTRIEVE], [CROSSREF], [CSA]
- Porsolt RD, Anton G, Blavet N, Jalfre M (1978): Behavioral despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol 47: 379–391, [INFOTRIEVE], [CROSSREF], [CSA]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I (2002): Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology 27: 303–318, [INFOTRIEVE], [CROSSREF], [CSA]
- Sapolsky RM (2000a): Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57: 925–935, [INFOTRIEVE], [CROSSREF], [CSA]
- Sapolsky RM (2000b): Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57: 925–935, [INFOTRIEVE], [CROSSREF], [CSA]
- Sapolsky RM (2000c): The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol. Psychiaty 15: 713–714, [CSA]
- Takeda H, Tsuji M, Matsumiya T (2000): Formation mechanisms of stress adaptation: Role of functional coupling of glucocorticoids and brain serotonergic nervous system. Nihon Shinkei Seishin Yakurigaku Zasshi 20: 83–91, [INFOTRIEVE], [CSA]
- Wong ML, Licinio J (2001): Research and treatment approaches to depression. Nat Rev Neurosci 2: 343–351, [INFOTRIEVE], [CROSSREF], [CSA]
- Yokoyama H, Hiai S, Oura H (1981): Chemical structures and corticosterone secretion-inducing activities of saikosaponins. Chem Pharm Bull (Tokyo) 29: 500–504, [CSA]