Abstract
In vitro. investigation of the antimicrobial activity of a crude methanol extract of leaves of Callistemon rigidus. R.Br. (Myrtaceae) revealed potential antibacterial activity against a broad spectrum of multidrug-resistant human pathogens. Agar well diffusion assays of the test extract established significant concentration-dependent antibacterial activity against methicillin-resistant Staphylococcus aureus., vancomycin-intermediate Staphylococcus aureus., vancomycin-resistant Escherichia coli., extended spectrum β.-lactamase-producing E. coli., and multidrug-resistant Pseudomonas. spp.
Introduction
Plant-derived medicines have been a part of traditional health care across the world for thousands of years (Chariandy et al., Citation1999). With the emergence and spread of antibiotic resistance in human pathogenic microorganisms, there is an increased need of screening natural products for newer and effective chemotherapeutic agents. Plants serve as a reservoir to numerous chemical entities, which are a part of the defense mechanism in response to microbial attack and adverse environmental conditions. These biologically active compounds exhibit antimicrobial properties (Cowan, Citation1999) and are largely unexplored (Hamburger & Hostettmann, Citation1991).
Callistemon rigidus. R.Br. (Myrtaceae) is a stiff upright shrub indigenous to tropical countries and Australia. Traditional medicinal systems advocate different medicinal properties of the plant. Leaves are used to cure cough, bronchitis, and other respiratory tract infections in Cameroon, Australia, China, and Asia (Jirovetz et al., Citation1997). Phytochemical analysis of the oil indicates the presence of mono- and sesquiterpenes, which could be responsible for their possible effects in folklore medicine (Jirovetz et al., Citation1997).
However, there are no experimental studies carried out on this plant to substantiate the folklore claims. This study was therefore designed to evaluate broad-spectrum antibacterial and anticandidal activity of the methanol extract of the leaves of Callistemon rigidus..
Materials and Methods
Plant collection
Fresh and healthy leaves (free from any visible contamination) were collected in the month of October at the Thapar Institute of Engineering and Technology campus by Ms. Charu Gomber and identified by Dr. Sanjai Saxena with confirmation from Punjab Horticulture Department. A specimen has been deposited at the herbarium of the Department of Biotechnology & Environmental Sciences and numbered as #7San03. Samples were thoroughly washed under running water and air-dried. The fresh weight was noted, and the samples were subjected to drying at 37°C and ground into coarse powder.
Extraction
Cold percolation using methanol as the solvent was used for extraction of the plant tissue. Pulverized plant material (70 g) was soaked in 150 ml of methanol in clean air-tight bottles. Extraction was done at 28°C, 120 rpm for 2 days. The solvent was filtered, and the extracts obtained were pooled to obtain the 7.5 g of residue. The residue was stored at −20°C until use.
Test microorganisms used
The test microorganisms used included Staphylococcus aureus. (31 isolates), Escherichia coli. (18 isolates), Pseudomonas. spp. (17 isolates), and Candida albicans. (6 isolates) (). The bacteria were maintained on trypticase soy agar slants and yeasts on Saboraud dextrose agar slants. Activation of cultures was carried out by streaking on a Mueller-Hinton (MH) agar plate followed by incubation overnight at 37°C. A single colony was picked from this plate and transferred to MH broth and incubated at 37°C prior to the test. In case of fungi, yeast extract peptone dextrose (YEPD) agar was used for activation. Susceptibility testing of all bacterial and fungal isolates was done (NCCLS, Citation2002; EARSS, Citation2005) based on cultures grouped as methicillin-resistant Staphylococcus aureus., vancomycin-resistant Staphylococcus aureus., vancomycin-intermediate Staphylococcus aureus., vancomycin-resistant E. coli., extended spectrum β.-lactamase-producing E. coli., and multidrug-resistant S. aureus. and Pseudomonas. spp. (). All isolates were resistant to one or more antibiotics. S. aureus. NCTC 6571 and E. coli. NCTC 10418 were included as reference strains.
Table 1 Culture collection.
Table 2 Culture classification based on susceptibility testing.
In vitro. antibacterial assays
Antibacterial activity of the test extract was determined by the agar well diffusion method (Reddish, Citation1929; Lehrer et al., Citation1991). The plant extract was dissolved in DMSO and tested at six different concentrations (16.65, 33.3, 66.6, 99.9, 199.8, and 399.9 µg). The assay was done by preparing 4 wells of 5 mm diameter per 90 mm MH agar plate (mean depth ± 4.00 mm) aseptically. Thirty microliters of the test extract at 6 different concentrations was dispensed in the test wells. Solvent blank was included as control. The plates were left for 15 min for diffusion after which the wells were sealed with molten MH agar. These were then swabbed with 18 h old, 0.5 McFarland adjusted culture of test isolates and incubated overnight at 37°C. Antibacterial activity was determined by measuring the zone of inhibition around the test wells. The growth inhibition diameter was an average of three different measurements.
Determination of anticandidal activity
This was performed using the agar well diffusion assay (as described for the determination of anticandidal activity) on YEPD agar.
Statistical analysis
Descriptive statistics using GraphPad Prism ver. 4.03 software was carried out for the assessment of effect of different parameters, that is, concentration of the extract and type of culture/isolate on the inhibitory activity (diameter of inhibition zone) was studied by two-way analysis of variance, that is, two-way ANOVA. Pearson correlation was calculated to assess the concentration-dependent antibacterial activity of the test extract against the bacterial isolates.
Results and Discussion
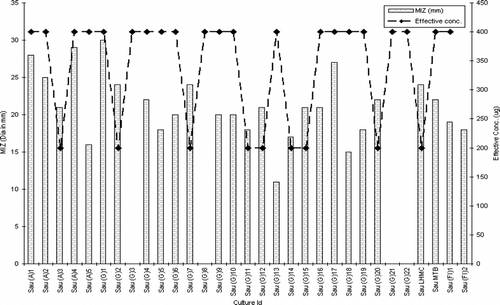
The extract was active against both Gram-positive and Gram-negative bacteria. There was no inhibitory activity in the control wells. Among Staphylococcus aureus. isolates, the extract exhibited strong activity against VISA, MRSA, and MARSA isolates, the majority of which gave an appreciable zone size (≥20.0 mm) at 199.8 µg. The exceptions to these were Sau (G) 8 and Sau (G) 21 that resisted the extract at all concentrations. The extract did not exhibit any activity against vancomycin-resistant strains [Sau (G) 3 and Sau (G) 22] ().
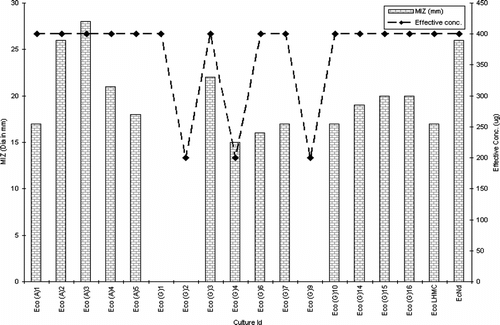
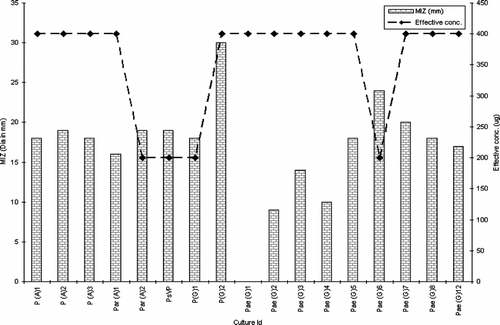
Among Gram-negative isolates, barring Eco (G) 2, the extract exhibited modest activity against all vancomycin-resistant E. coli. (VRE). Similarly, excluding Eco (G) 1 and Eco (G) 3, the extract displayed potential activity against extended spectrum β.-lactamase-producing strains (). Tests against Pseudomonas. species depict the killing effect of the extract against all isolates excluding Pae (G) 1, Pae (G) 3, and Pae (G) 4 (). The extract was not active against any of the test isolates of Candida albicans..
Agar well diffusion is a popular prescreen employed by clinical microbiologists, pharmacognosists, and phytochemists working on antimicrobial drug development from plants (Nakamura et al., Citation1999; Alves et al., Citation2000). Antimicrobial activity of numerous plants has been evaluated using agar well diffusion assay (Hoffmann et al., Citation2004; Guven et al., Citation2005; Okoli & Iroegbu, Citation2005). The current work is the first scientific documentation of the antimicrobial potential of Callistemon rigidus. against multidrug-resistant bacteria.
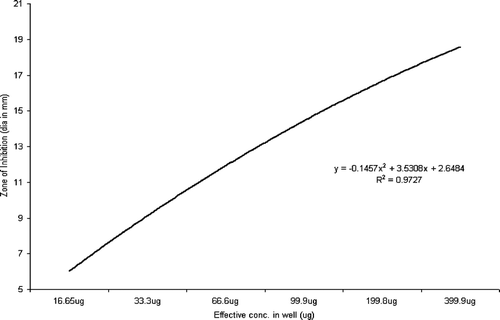
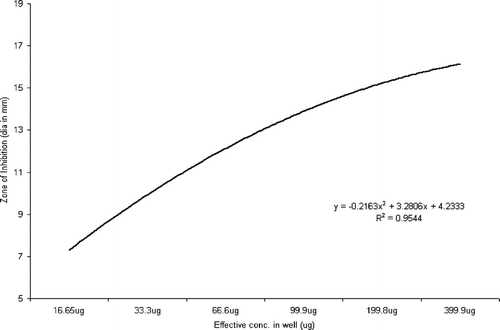
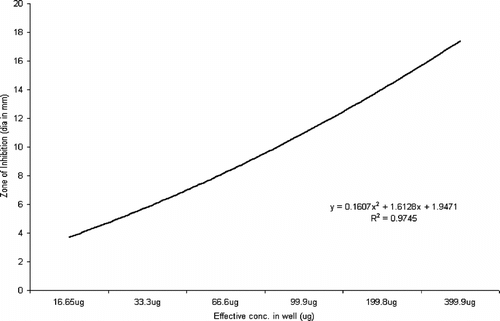
Statistical analysis using ANOVA revealed a significant effect (p < 0.0001) of the test parameters, viz., isolate type and concentration of extract on the inhibition zone (Rambali et al., Citation2001; Zelenitsky et al., Citation2003; Larabi et al., Citation2004). Pearson correlation is a very important parameter in understanding the dose-drug behavior as well as drug efficacy in terms of post antibiotic effect (Stubbings et al., Citation2004). There was a highly significant correlation between the dose of the extract and diameter of inhibition zone against all the three groups of test isolates. The R2 value of drug concentration to inhibition zone diameter was 0.9745 in Pseudomonas. species, followed by 0.92727 in Staphylococcal. isolates and 0.9544 in Escherichia. species (, respectively).
Conclusion
The current investigation is the first experimental documentation of the antimicrobial efficacy of leaf extract of Callistemon rigidus.. The in vitro. antimicrobial activity of the methanol extract of leaves of Callistemon rigidus. against all isolates (predominantly toward MRSA, MARSA, ESBL, and VISA strains) signifies that bioactive compounds may be present. Preliminary isolation with column chromatography and further antibacterial testing has confirmed the presence of single active compounds in the methanol extract of leaves of Callistemon rigidus.. Currently, work is being conducted to further isolate and identify these active constituents.
Acknowledgment
We are grateful to Dr. Rajendra Prasad, Department of Life Sciences, Jawaharlal Nehru University, New Delhi, Dr. Aarti Kapil, Department of Microbiology, All India Institute of Medical Sciences, Prof. S. Arvidson, Microbiology & Tumor Biology Centre, Karolinska Institute, Sweden, and Dr. Amarjit Kaur Gill, Department of Microbiology, Government Medical College, Patiala for providing us multidrug-resistant cultures of S. aureus., E. coli, Pseudomonas., and C. albicans.. We acknowledge Dr. S. Natesh and Dr. M. Aslam for their continuous inspiration for the current work, and Charu Gomber acknowledges DBT, Government of India, for providing a fellowship under the project number BT/PR/3982/PBD/17/257/2003.
References
- Alves TMA, Silva AF, Brandão M, Grandi TSM, Smânia EF, Smânia A Jr, Zani CL (2000): Biological screening of Brazilian medicinal plants. Mem Inst Oswaldo Cruz 95: 367–373, [INFOTRIEVE], [CSA]
- Chariandy CM, Seaforth CE, Phelps RH, Pollard GV, Khambay BPS (1999): Screening of medicinal plants from Trinidad and Tobago for antimicrobial and insecticidal properties. J Ethnopharmacol 64: 265–270, [INFOTRIEVE], [CROSSREF], [CSA]
- Cowan MM (1999): Plant products as antimicrobial agents. Clin Microbiol Rev 12: 564–582, [INFOTRIEVE], [CSA]
- European Antimicrobial Resistance Surveillance System (2005): New and Updated protocols for antimicrobial susceptibility testing of pathogens under EARSS surveillance 2005. (Available online at www.earss.rivm.nl).
- Guven K, Celik S, Uysal I (2005): Antimicrobial activity of Centaurea. species. Pharm Biol 43: 67–71, [CSA]
- Hamburger M, Hostettmann K (1991): Bioactivity in plants: The link between phytochemistry and medicine. Phytochemistry 30: 3864–3874, [CROSSREF], [CSA]
- Hoffman BR, DelasAlas H, Blanco K, Wiederhold N, Lewis RE, Williams L (2004): Screening of antibacterial and antifungal activities of ten medicinal plants from Ghana. Pharm Biol 42: 13–17, [CSA]
- Jirovetz L, Fleischhacker W, Buchbauer G, Ngassoum MB (1997): Analysis of the essential oils of Callistemon rigidus. (Myrtaceae) from Cameroun by GC/FID and GC/MS. Sci Pharm 65: 315–319, [CSA]
- Larabi M, Pages N, Pons F, Appel M, Gulik A, Schlatter J, Bouvet S, Barratt G (2004): Study of the toxicity of a new lipid complex formulation of amphotericin B. J Antimicrob Chemother 53: 81–88, [INFOTRIEVE], [CROSSREF], [CSA]
- Lehrer RI, Rosnaman M, Harwig SS, Jackson R, Eisenhauer P (1991): Ultrasensitive assays for endogenous antimicrobial polypeptides. Immunol Methods 137: 167–173, [CROSSREF], [CSA]
- Nakamura CV, Ueda Nakamura T, Bando E, Melo AFN, Cortez DAG, Dias Filho BP (1999): Antibacterial activity of Ocimum gratissimum. L. essential oil. Mem Inst Oswaldo Cruz 94: 675–678, [INFOTRIEVE], [CSA]
- NCCLS (National Committee for Clinical Laboratory Standards) (2002): Performance standards for antimicrobial disc susceptibility tests. Document no. NCCLS M100-S12, NCCLS, Wayne, PA, USA.
- Okoli S, Iroegbu CU (2005): In vitro. antibacterial activity of Synclisa scabrida. whole root extracts. Afr J Biotechnol 4: 946–952, [CSA]
- Rambali B, Fernandez JA, Nuffle LV, Woestenborghs F, Baert L, Massart DL, Odds FC (2001): Susceptibility testing of pathogenic fungi with itraconazole: A process analysis of variables. J Antimicrob Chemother 48: 163–177, [INFOTRIEVE], [CROSSREF], [CSA]
- Reddish, GF (1929): Methods of testing antiseptics. J Lab Clin Med 14: 649–658, [CSA]
- Stubbings WJ, Bostock JM, Ingham E, Chopra I (2004): Assessment of a microplate method for determining the post-antibiotic effect in Staphylococcus aureus. and Escherichia coli.. J Antimicrob Chemother 54: 139–143, [INFOTRIEVE], [CROSSREF], [CSA]
- Zelenitsky SA, Harding GKM, Sun S, Ubhi K, Ariano RE (2003): Treatment and outcome of Pseudomonas aeruginosa. bacteraemia: An antibiotic pharmacodynamic analysis. J Antimicrob Chemother 52: 668–674, [INFOTRIEVE], [CROSSREF], [CSA]