Abstract
DNA topoisomerases are essential enzymes that regulate the conformational changes in DNA topology by catalyzing the concerted breakage and rejoining of DNA strands during normal cellular growth. During the past few years, there has been considerable pharmacological interest in these enzymes because the inhibitors of DNA topoisomerases represent a major class of anticancer drugs. In this study, we investigated the effects of the extracts, prepared from a number of Helichrysum. taxa, on mammalian DNA topoisomerase I via in vitro. supercoil relaxation assays using plasmid substrate, pBR322. Cytotoxic alkaloid, camptothecin, an inhibitor of eukaryotic topoisomerase I, was used as reference compound throughout the assays. Quantitative comparisons of the supercoiled and relaxed DNA band intensities on agarose gels suggested that the extracts from H. pallasii. (Sprengel) Ledeb., H. armenium DC subsp.. araxinum. (Kirp.) Takht, and H. plicatum DC subsp.. plicatum. manifested a considerable inhibition on the enzyme in a dose-dependent manner. The inhibition of mammalian DNA topoisomerase I by Helichrysum. extracts is a significant result because they can be potential sources of anticancer drugs.
Introduction
Identifying plant species with abundant quantities of antiproliferative agents that interfere with the functioning of cellular DNA to prevent, slow, and/or reverse the cancer induction is one of the mainstays of cancer chemotherapy. Prominent among the anticancer agents are the general class known as the DNA topoisomerase inhibitors. DNA topoisomerases are essential enzymes that regulate the conformational changes in DNA topology by cleaving and religating either one (type I) or two (type II) strands of DNA thereby allowing these strands to pass through one another (Wang, Citation1996). Many reports in recent years have shown that numerous naturally occurring and synthetic compounds have the ability to interfere with normal topoisomerase function (Topcu, Citation2001; Denny, Citation2004). A number of these compounds were in clinical use in the 1970s before their cytotoxic actions were linked to topoisomerases. Examples are fluoroquinolone antibacterial agents (Lesher et al., Citation1962) and camptothecin (CPT) (Wall et al., Citation1966).
The genus Helichrysum. is a large group of plants that is represented by 27 taxa belonging to 21 species in Turkish flora, out of which 15 are endemic to Turkey (available at International Herbarium of Ege University, Faculty of Pharmacy, IZEF, Izmir, Turkey; http://www.izef.ege.edu.tr) (Cubukcu & Mericli, Citation1977; Cubukcu & Yuksel, Citation1982; Cubukcu & Bingol, Citation1984). Several Helichrysum. plants were shown to contain antioxidant (Czinner et al., Citation2000; Tepe et al., Citation2005), antifungal (Barberán et al., Citation1988), antibacterial and antiviral bioactive compounds (Meyer et al., Citation1996Citation1997). This group of plants is also known to be rich in flavonoids. About 20 flavonoids have been identified previously as topoisomerase inhibitors (Webb & Ebeler, Citation2004; Ma et al., Citation2005). As part of our work in the search for biologically active compounds, we surveyed a number of selected Helichrysum. extracts for their ability to interfere with mammalian DNA topoisomerase I via in vitro. supercoil relaxation assays using plasmid substrate, pBR322.
Materials and Methods
Plant material
Helichrysum. species were collected at the flowering period from various locations in the years 2000 and 2001. Voucher specimens were identified and deposited at IZEF. shows the locales of the species screened in our study.
Table 1 Localities and the extraction yield of the collected Helichrysum. species.
Preparation of methanol extracts
The capitules of Helichrysum. taxa were dried in shade. Five grams of flowering tops were suspended in 100 ml of 70% methanol and extracted under reflux for 3 h (Carini et al., Citation2001). Insoluble residue was re-extracted twice using the same solvent and both extracts combined. The solvent was then evaporated under vacuum to dryness at 60°C, and yellow-colored extracts were collected. The dried extracts were re-dissolved in dimethylsulfoxide (DMSO).
Plasmid supercoil relaxation assay
Plasmid supercoil relaxation assays were carried out as described (Topcu & Castora, Citation1995). Briefly, 20 µl of reaction mixture contained one unit of calf thymus topoisomerase I, 0.5 µg of supercoiled (sc) pBR322 (TAKARA, Otsu-Shiga, Japan), in the presence or absence of the extracts in 35 mM Tris-HCl, (pH 8.0), 72 mM KCl, 5 mM MgCl2, 5 mM DTT, 5 mM spermidine, and 0.1% bovine serum albumin. A stock solution of 10 µg/mL CPT in DMSO was serially diluted for comparisons. The relaxation products were analyzed on 1% agarose gels in TBE buffer (45 mM Tris borate and 1 mM EDTA, pH 8.0) in a horizontal electrophoresis apparatus (5 V/cm) (Thermo EC250) and photographed under UV light after staining in ethidium bromide (EtdBr) solution (0.5 µg/ml). The relationship between the binding of EtdBr and the amount of fluorescence given by sc and relaxed DNA (rlx DNA) under UV light was carried out as described (Topcu, Citation2000). DNA bands were quantified from gel photographs using BioRad Multianalyst (ver. 1.1). One unit of the enzyme activity was defined as the activity removing the supercoils from 500 ng of sc plasmid substrate at 37°C in 30 min. All reactions were carried out in DNase-free 1.5 ml microcentrifuge tubes.
Statistical evaluation
Statistical evaluation of the inhibitions was carried out using two-tailed combined correlation analyses.
Results and Discussion
Our assay employs sc plasmid DNA and relies on the ability of topoisomerase I to relax sc DNA, which can be separated as discrete bands using gel electrophoresis. An inhibition of relaxation activity due to the presence of a particular extract is monitored as the accumulated faster-migrating supercoiled DNA. In addition to visual inspection of the effects of the plant extracts on the gels, we made a quantitative and standardized measure of these effects for meaningful comparisons between different compounds.
We screened methanolic extracts from capitules of 34 samples of three Helichrysum. taxa through in vitro. plasmid supercoil relaxation assays of mammalian DNA topoisomerase I using CPT as positive control. The overall yield in Helichrysum. extracts that resulted in detectable inhibition of supercoil relaxation was between 9.1% and 22.4% ().
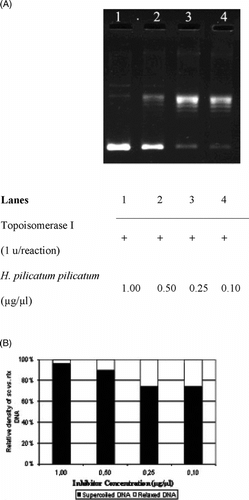
The interference in the supercoil relaxing activity of DNA topoisomerase I in the presence of varying concentrations of H. pallasii. is shown in . As seen in , the sc DNA (, lane 1) was fully relaxed by the enzyme (, lane 2). The organic solvent, DMSO, did not exert any detectable inhibition on the DNA topoisomerase I activity in the identical experimental conditions (, lane 3). Relaxation of sc plasmid substrate, pBR322, was inhibited upon incubation with the extract (, lanes 4 to 7), and the inhibition was diminished at the concentration of 0.05 µg/µl, as monitored by the accumulation of faster-migrating sc pBR322 (, lane 8). Densitometric quantification of the remaining sc plasmid band intensities is shown in . The same experiments were carried out using H. armenium araxinum. () and H. plicatum plicatum. () extracts. The inhibition by the extract of H. armenium araxinum. was less profound compared with H. pallasii. because the 0.25 µg/ml dilution (, lane 5) resulted in a comparable distribution of sc and rlx DNA bands obtained in the reaction in the absence of the extract (, lane 2). A comparable inhibition pattern to H. armenium araxinum. was seen with H. plicatum plicatum. (). The densitometric quantifications of the percent inhibitions manifested by the H. armenium araxinum. and H. plicatum plicatum. are shown in and , respectively.
Figure 1 Interfering activities of selected Helichrysum. taxa on DNA topoisomerase I. (A) Agarose gel photograph of the plasmid supercoil relaxation assays in the presence of varying concentrations of H. pallasii.. Lane 1, pBR322 plasmid DNA without enzyme; lane 2, supercoil relaxation with 1 unit of DNA topoisomerase I; lane 3, same as lane 2 in the presence of DMSO; lanes 4–9; same as lane 2 in the presence of decreasing concentrations of H. pallasii. extracts (1.0 to 0.01 µg/µl). (B) Quantitative assesment of dose-dependence of the inhibition obtained with H. pallasii.. The percent of sc versus rlx DNA density is shown for each bar. See “Materials and Methods” for the details of sample treatments and gel electrophoresis conditions.
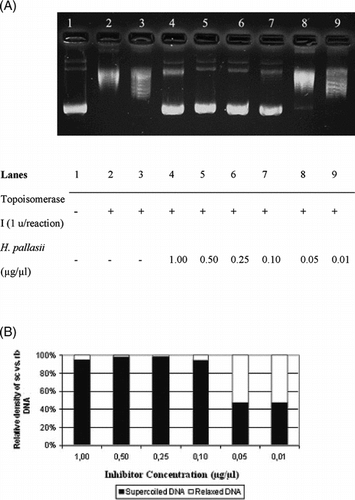
Figure 2 Interfering activities of selected Helichrysum. taxa on DNA topoisomerase I. (A) Agarose gel photograph of the plasmid supercoil relaxation assays in the presence of varying concentrations of H. armenium araxinum.. Lane 1, pBR322 plasmid DNA without enzyme; lane 2, supercoil relaxation with 1 unit of DNA topoisomerase I; lanes 3–6; same as lane 2 in the presence of decreasing concentrations of H. pallasii. extracts (1.0 to 0.1 µg/µl). (B) Quantitative assesment of dose-dependence of the inhibition obtained with H. armenium araxinum.. The percent of sc versus rlx DNA density is shown for each bar. See “Materials and Methods” for the details of sample treatments and gel electrophoresis conditions.
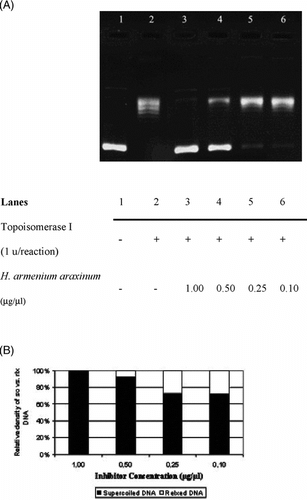
Figure 3 Interfering activities of selected Helichrysum. taxa on DNA topoisomerase I. (A) Agarose gel photograph of the plasmid supercoil relaxation assays in the presence of varying concentrations of H. plicatum plicatum.. Lanes 1–4, supercoil relaxation with 1 unit of DNA topoisomerase I in the presence of decreasing concentrations of H. plicatum plicatum. extracts (1.0 to 0.1 µg/µl). (B) Quantitative assesment of dose-dependence of the inhibition obtained with H. plicatum plicatum.. The percent of sc versus rlx DNA density is shown for each bar. See “Materials and Methods” for the details of sample treatments and gel electrophoresis conditions.
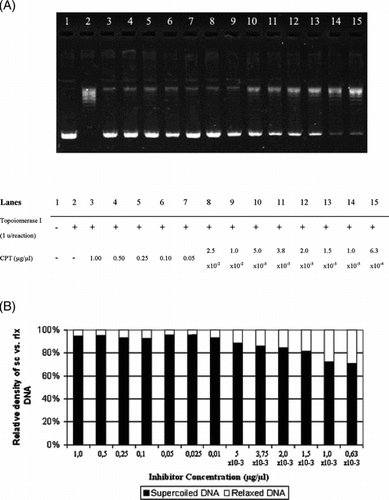
Next, we compared the inhibitory activities of Helichrysum. extracts with the well-known DNA topoisomerase I inhibitor, CPT (). Being a pure compound, CPT manifested a more profound inhibition both in comparable (, lanes 3 to 6) and diluted concentrations (, lanes 7 to 13) relative to the extract concentrations, which was also verified quantitatively (). However, the inhibition at the higher concentrations of CPT (1.00 to 0.03 µg/µl) was abundant but less precise when compared with lower CPT dilutions (0.01 to 0.6 × 10−3 µg/µl) ().
Figure 4 Interfering activity of CPT on DNA topoisomerase I. (A) Agarose gel photograph of the plasmid supercoil relaxation assays in the presence of varying concentrations of CPT. Lane 1, pBR322 plasmid DNA without enzyme; lane 2, supercoil relaxation with 1 unit of DNA topoisomerase I; lanes 3–15; same as lane 2 in the presence of decreasing concentrations of CPT (1.0 to 6.0 × 10−4 µg/µl). (B) Quantitative assesment of dose-dependence of the inhibition obtained with CPT. The percent of sc versus rlx DNA density is shown for each bar. See “Materials and Methods” for the details of sample treatments and gel electrophoresis conditions.
Among the three different taxa, the inhibition obtained using H. pallasii. was considerable compared with the inhibition obtained using CPT standards; therefore, this extract can be categorized as a strong inhibitor relative to H. armenium araxinum. and H. plicatum plicatum. extracts (). The latter two extracts resulted in intermediate inhibitions ( and ). Indeed, this subgrouping was supported by the statistical evaluation. When the quantitative values of the inhibitions by the three extracts were compared with CPT inhibition with two-tailed combined correlation analyses, H. pallasii. had a significance level (ρ) of 0.650, while the other two extracts H. armenium araxinum. and H. plicatum. had significance levels of 0.116 and 0.145, respectively. Given that the components of the Helichrysum. species are variable among the subspecies (Cubukcu & Bingol, Citation1984), the difference in the strength of inhibition among the Helichrysum. extracts would, possibly, be due to the difference in the percent compositions of the compounds constituting their chemical compositions (Kilinc et al., unpublished data).
Taken together, this is the first study reporting an inhibitory effect of Helichrysum. species on mammalian DNA topoisomerase I. Inhibitors of topoisomerases act at any of three major steps in the mechanism of action of the enzymes; the enzyme binding to DNA, the breakage of DNA strands, or re-ligation of the DNA following the strand passing step (Topcu, Citation2001). Flavonoids and other polyphenolic compounds have been shown to inhibit human topoisomerase I through both inhibition of relaxation activity and stabilization of the cleavable complex (poisoning) (Ferguson, Citation2001; Webb & Ebeler, Citation2004). Some flavonoids have also been shown to intercalate DNA, and an association of topoisomerase inhibition with intercalation has been reported (Webb & Ebeler, Citation2004). Studying the mechanism of the inhibition by the Helichrysum. extracts needs the identification and quantification of the chemical components of the extracts, which is currently in progress. Considering the rich flora of Turkey and the geographical distribution of Helichrysum. species, the inhibition we obtained is a significant result because these plants can be potential sources of anticancer agents.
Acknowledgments
The authors acknowledge Dr. O. Sercan, Dr. O. Secmen, and Dr. U. Zeybek for their valuable help at various steps of this study, and Dr. M. Tosun for carefully reading the manuscript.
References
- Barberan FAT, Msonthi JD, Hostettmann K (1988): Antifungal epicuticular methylated flavonoids from Helichrysum nitens.. Phytochemistry 27: 753–755, [CSA]
- Carini M, Aldini G, Furlanetto S, Stefani R, Facino RM (2001): LC couplet to ion trap MS for the rapid screening and detection of polyphenol antioxidants from Helichrysum stoechas.. J Pharm Biomed Anal 24: 517–526, [INFOTRIEVE], [CROSSREF], [CSA]
- Cubukcu B, Bingol S (1984): Pharmacognostical investigations on Helichrysum pallasii. (Sprengel) Ledeb. Plantes Medicinales et Phytotherapie 18: 28–35, [CSA]
- Cubukcu B, Mericli AH (1977): Flanoides D'Helichrysum plicatum DC. Plantes Medicinales et Phytotherapie 11: 294–302, [CSA]
- Cubukcu B, Yuksel V (1982): Constituens of Anatolian medicinal plants: Flavonoids of Helichrysum armenium.. J Nat Prod 45: 137–139, [CROSSREF], [CSA]
- Czinner E, Hagymasi K, Blazovics A, Kery A, Szöke E, Lemberkovics E (2000): In vitro. antioxidant properties Helichrysum arenarium. (L.) Moench. J Ethnopharmacol 73: 437–443, [INFOTRIEVE], [CROSSREF], [CSA]
- Denny WA (2004): Emerging DNA topoisomerase inhibitors as anticancer drugs. Expert Opin Emerg Drugs 9: 105–133, [INFOTRIEVE], [CSA]
- Ferguson LR (2001): Role of plant polyphenols in genomic stability. Mutat Res 475: 89–111, [INFOTRIEVE], [CSA]
- Lesher GY, Froelich EJ, Gruett MD, Bailey JH, Brundage RP (1962): 1-Naphtyridine derivatives. A new class of chemotherapeutic agents. J Med Chem 26: 1063–1065, [CROSSREF], [CSA]
- Ma J, Jones SH, Marshall R, Wu X, Hecht SM (2005): DNA topoisomerase I inhibitors from Rinorea anguifera.. Bioorg Med Chem Lett 15: 813–816, [INFOTRIEVE], [CROSSREF], [CSA]
- Meyer JJM, Afolajan AJ, Taylor MB, Engelbrecht L (1996): Antibacterial activity of Helichrysum aureonitens. (Asteraceae). J Ethnopharmacol 52: 41–43, [INFOTRIEVE], [CROSSREF], [CSA]
- Meyer JJM, Afolajan AJ, Taylor MB, Erasmus D (1997): Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens.. J Ethnopharmacol 56: 165–169, [INFOTRIEVE], [CROSSREF], [CSA]
- Tepe B, Sokmen M, Akpulat HA, Sokmen A (2005): In vitro antioxidant activities of the methanol extracts of four Helichrysum. species from Turkey. Food Chem 30: 685–689, [CROSSREF], [CSA]
- Topcu Z (2000): Densitometric quantification of DNA topoisomers in ethidium bromide-stained agarose gels and chemiluminescence-detected X ray films. Acta Biochim Polonica 47: 835–839, [CSA]
- Topcu Z (2001): DNA topoisomerases as targets for anticancer drugs. J Clin Pharm Ther 26: 405–416, [INFOTRIEVE], [CROSSREF], [CSA]
- Topcu Z, Castora FJ (1995): Mammalian mitochondrial DNA topoisomerase I preferentially relaxes supercoils in plasmids containing specific mitochondrial DNA sequences. Biochim Biophys Acta 1264: 377–387, [INFOTRIEVE], [CSA]
- Wall M, Wani MC, Cooke CE, Palmer KH, McPhail AT, Sim GA (1966): Plant anti-tumor agents I: The isolation and structure of camptothecin, a novel alkaloidal leukemia and antitumor inhibitor from Camptotheca acuminata.. J Am Chem Soc 26: 3888–3890, [CROSSREF], [CSA]
- Wang JC (1996): DNA topoisomeases. Ann Rev Biochem 65: 635–692, [INFOTRIEVE], [CROSSREF], [CSA]
- Webb MR, Ebeler SE (2004): Comparative analysis of topoisomerase IB inhibition and DNA intercalation by flavonoids and similar compounds: Structural determinates of activity. Biochem J 384: 527–541, [INFOTRIEVE], [CROSSREF], [CSA]
