Abstract
Fractions from a partition of a total extract of Hypericum ascyron. L. and Hypericum japonicum. Thunb. ex Murray were investigated for their antibacterial activity against a strain of multidrug-resistant (MDR) Staphylococcus aureus. possessing the NorA multidrug-efflux transporter, the major characterized MDR pump in this species. The hexane fraction of Hypericum ascyron. and the n.-BuOH fraction of H. japonicum. both showed appreciable antibacterial activities with minimum inhibitory concentration (MIC) values of 128 µg/ml for both fractions. Given the complexity of these extracts and the relevance of the NorA efflux mechanism in clinical isolates of Staphylococcus aureus., these results highlight the potential of the genus Hypericum. as a source of new antibacterial drug-leads.
Introduction
As part of a continuing project to characterize plant-derived antibacterial natural products from the genus Hypericum. (Gibbons et al., Citation2002Citation2005), we have evaluated two Chinese species of this group (H. ascyron L... and H. japonicum. G. Thunb. ex Murray) against a multidrug-resistant strain of Staphylococcus aureus.. This work was started due to the exquisite activity of hyperforin toward effluxing strains of staphylococci, which in many cases had minimum inhibitory concentration values of less than 1 µg/ml against multidrug-resistant (MDR) strains (Gibbons, unpublished results). In the traditional Chinese medicine system, Hypericum ascyron. (“hong han lian” or “han lian” herb) is used to remove toxicity and to cool the blood of the human body. Hypericum japonicum. is used as an herbal medicine to treat hepatitis B and carbuncles in China (JSNMC, Citation1986; Zhang, Citation1993; Wu, Citation1998). We have demonstrated that many extracts of Hypericum. plants possess antibacterial activity toward Staphylococcus aureus. (Gibbons et al., Citation2002), and the metabolites responsible for these activities are likely to be prenylated acylphloroglucinol natural products related to hyperforin (Gibbons, Citation2004). Xanthones, flavonoids, flavone glycosides, and acylphloroglucinol natural products have been isolated from the two species in previous research (Wang et al., Citation1980; Ishiguro et al., Citation1987Citation1990Citation2002; Chen et al., Citation1992; Wu et al., Citation1998; Hu et al., Citation2000). In this paper, we report the inhibitory effects of extracts of both species against a strain of multidrug-resistant Staphylococcus aureus..
Materials and Methods
Plant material
The whole herb of Hypericum ascyron. was purchased from a local Chinese traditional medicinal materials store in Hunan province, China. The herb of H. japonicum. was purchased from the Medicinal Material Company (Shanghai, China). Voucher specimens for both species have been deposited at the pharmacognosy laboratory of the School of Pharmacy, Fudan University.
Extraction and fractionation
Dried herbal material of H. ascyron. (150.0 g) was chopped and extracted in 95% ethanol (400 ml × 3) at 45°C. Extraction with hexane (50 ml × 3) and evaporation of the extract gave HA01, the hexane-soluble fraction, together with HA02, an insoluble portion. The latter (HA02) was dissolved in chloroform (50 ml × 3), and the soluble fraction was denoted as HA03. Addition of distilled water (50 ml × 2), followed by suspension and sequential centrifugation afforded a residue (HA04). Freeze-drying of the supernatant afforded fraction HA05. Samples HJ01–HJ05 were obtained using the same method for the herbal material of H. japonicum. (50.0 g).
Thin-layer chromatography
The above fractions of H. ascyron. and H. japonicum. were subjected to thin-layer chromatography (TLC). The silica TLC precoated plates were made in the Qingdao Marine Chemical Plant (Qingdao, China), and the RP-18 plates were purchased from E. Merck Co. Ltd. (Shanghai, China). The developed TLC plates were colored with 20% H2SO4 followed by heating.
Bacteria
SA-1199B is a strain of Staphylococcus aureus. overproducing the NorA MDR efflux protein, the major drug pump in S. aureus., and was resistant to norfloxacin (MIC = 32 µg/ml). Additionally, some of this resistance is a result of a GrlA subunit substitution known to correlate with diminished fluoroquinolone susceptibility (Kaatz & Seo, Citation1997).
Determination of minimum inhibitory concentration (MIC)
Norfloxacin was obtained from the Sigma Chemical Co. Mueller-Hinton broth (MHB; Oxoid, Basingstoke, UK) was adjusted to contain 20 mg/l Ca2+ and 10 mg/l Mg2+.Overnight cultures of the strain were made up in 0.9% saline to an inoculum density of 5 × 105 cfu/ml by comparison with a McFarland standard. Norfloxacin and extracts were dissolved in DMSO and then diluted in MHB to give a starting concentration of 512 µg/ml. Using Nunc 96-well microtiter plates, 125 µl of MHB was dispensed into wells 1–11; 125 µl of the extract or norfloxacin solution was dispensed into well 1 and serially diluted across the plate leaving well 11 empty for the growth control. The final volume was dispensed into well 12, which being free of MHB or inoculum served as the sterile control. Finally, the bacterial inoculum (125 µl) was added to wells 1–11 and the plate was incubated at 37°C for 18 h. A DMSO control (3.125%) was included. All MICs were determined in duplicate. The MIC was determined as the lowest concentration at which no growth was seen. A methanol solution (5 mg/ml) of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Lancaster) was used to detect bacterial growth by a color change from yellow to blue.
Results and Discussion
The hexane fraction of the H. ascyron. extract (HA01) and the HJ04 fraction of the H. japonicum. extract were both active and inhibited the growth of SA-1199B at a concentration of 128 µg/ml. For norfloxacin, the minimum inhibitory concentration was 32 µg/ml. Surprisingly, this activity was confined to only two fractions of the whole extract, as other fractions were inactive at 128 µg/ml. Although these MIC values are higher than the standard control, they are encouraging because TLC analysis () of these fractions indicates that they are complex with a number of bands present that “streak” and are poorly resolved. This would indicate that there might be active compounds present in the fractions with sub 1 µg/ml MIC values. Furthermore, the activity demonstrated is against a clinical isolate possessing the NorA efflux protein, the major characterized antibiotic pump in S. aureus., and this indicates that the compounds present are possibly not substrates for this efflux system. This would be an obvious advantage in an antibacterial drug-lead candidate, as multidrug efflux is becoming more commonly detected in clinical isolates (Lage, Citation2003).
Figure 1 Thin-layer chromatograms of HA and HJ fractions. Samples in plates (A) and (B): HA fractions: 1, HA crude extract; 2, HA01 (hexane fraction); 3, HA03 (chloroform fraction); 4, HA04. TLC conditions: (A) petroleum:acetone (8:2); (B) CHCl3:MeOH (99:1); H2SO4 sprayed followed by heating. Samples in plates (C) and (D) HJ fractions: 1, HJ01 (hexane fraction); 2, HJ03 (chloroform fraction); 3, HJ04. TLC conditions: (C) EtOAc:MeOH (96:4); (D) EtOAc:MeOH (7:3); H2SO4 sprayed followed by heating.
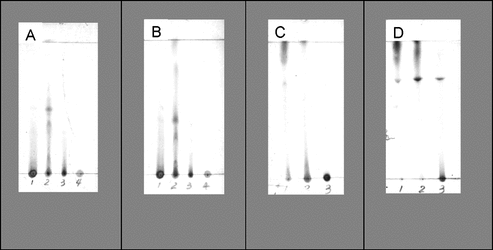
The active fraction of H. ascyron. was the hexane fraction HA01, a greenish and wax-like solid, which possessed extreme polarity in the TLC development (). The other species, H. japonicum., showed activity associated with HJ04 that was red-brown in color and microcrystalline. Even though the polar crystals of HJ04 were soluble in methanol, it was poorly resolved on normal phase silica plates employing a polar solvent system (EtOAc:MeOH, 7:3, ) or reversed phase RP-18 plates (MeOH:H2O, 65:35). This would indicate that there are compounds present that interact with silanols of the silica or uncapped silanols of the reverse phase sorbent.
Bioassay-guided isolation of the extracts of H. ascyron. and H. japonicum. are needed to isolate and characterize the bioactive compounds responsible for the observed activity, and these studies are now underway.
Acknowledgments
This work was supported by the National Science and Foundation of China (30472068). Professor Glenn W. Kaatz is thanked for the generous gift of bacterial strain SA-1199B.
References
- Chen JY, Lin CC, Namba T (1992): Development of natural crude drug resources from Taiwan (X). Pharmacognostical studies on the Chinese crude drug “han-lian-cao” (H. ascyron.). Am J Chin Med 20: 51–64, [INFOTRIEVE], [CSA]
- Gibbons S, Ohlendorf B, Johnsen I (2002): The genus Hypericum.—a valuable resource of anti-Staphylococcal leads. Fitoterapia 73: 300–304, [INFOTRIEVE], [CROSSREF], [CSA]
- Gibbons S (2004): Anti-staphylococcal plant natural products. Nat Prod Rep 21: 263–277, [INFOTRIEVE], [CROSSREF], [CSA]
- Gibbons S, Moser E, Hausmann S, Stavri M, Smith E, Clennett C (2005): An anti-staphylococcal acylphloroglucinol from Hypericum foliosum.. Phytochemistry 66: 1472–1475, [INFOTRIEVE], [CROSSREF], [CSA]
- Hu LH, Khoo CW, Vittal JJ, Sim KY (2000): Phloroglucinol derivatives from Hypericum japonicum.. Phytochemistry 53: 705–709, [INFOTRIEVE], [CROSSREF], [CSA]
- Ishiguro K, Yamaki M, Kashihara M, Takagi S (1987): Saroaspidin A, B, and C: Additional antibiotic compounds from Hypericum japonicum.. Planta Med 53: 415–417, [INFOTRIEVE], [CSA]
- Ishiguro K, Yamaki M, Kashihara M, Takagi S, Isoi K (1990): Sarothralin G: A new antimicrobial compound from Hypericum japonicum.. Planta Med 56: 274–276, [INFOTRIEVE], [CSA]
- Ishiguro K, Nagata S, Oku H, Yamaki M (2002): Bisxanthones from Hypericum japonicum.: Inhibitors of PAF-induced hypotension. Planta Med 68: 258–261, [INFOTRIEVE], [CROSSREF], [CSA]
- JSNMC (Jiang Su New Medical College) (1986): Dictionary of Chinese Materia Medica. Shanghai, Shanghai Science and Technology Publisher, pp. 813–1002.
- Kaatz GW, Seo SM (1997): Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus.. Antimicrob Agents Chemother 41: 2733–2737, [INFOTRIEVE], [CSA]
- Lage H (2003): ABC-transporters: Implications on drug resistance from microorganisms to human cancers. Int J Antimicrob Agents 22: 188–199, [INFOTRIEVE], [CROSSREF], [CSA]
- Wang ZQ, Wang XR (1980): Studies on the constituents of hong han lian (Hypericum ascyron. L.). Yao Xue Xue Bao 15: 365–367 (Chinese), , [INFOTRIEVE], [CSA]
- Wu ZY (1998): Compendium of New China (Xinhua) Herbal. Shanghai, Shanghai Science and Technology Publisher, pp. 214–216.
- Wu QL, Wang SP, Du LJ, Yang JS, Xiao PG (1998): Xanthones from Hypericum japonicum. and H. henryi.. Phytochemistry 49: 1395–1402, [INFOTRIEVE], [CROSSREF], [CSA]
- Zhang GJ (1993): Atlas of Practical Identification of Chinese Traditional Medicinal Materials. Hei Long Jiang, China Hei Long Jiang Science and Technology Press, pp. 305–362, 916.
