Abstract
Cassia angustifolia. Vahl (Leguminosae), commonly known as “sanaai,” is employed in various indigenous systems of medicine against several diseases, and almost every part of the plant has diverse medicinal properties. The seeds are used as an anthelmintic, digestive, and to treat piles, skin diseases, and abdominal troubles. According to Ayurveda, it has the property of reducing “kapha” and “Vata.” The current communication provides a detailed account of the pharmacognostic investigation carried out on the seeds of C. angustifolia.. The study includes macro- and microscopical details, SEM studies, fluorescence study of powder, physicochemical studies, and HPTLC fingerprinting. The seed is characterized by a finely ridged seed coat and palisade-like malpighian cells, discontinuous transparent linea lucida in the upper half of the malpighian layer, hilum simple and oblong. The study revealed that the seed samples procured from different places have similar morphological and physicochemical values. These observations are supported by TLC profiles. It was noted that the percentage of active principles (sennoside A and B) varied significantly in samples procured from different parts of the country.
Introduction
Cassia angustifolia. Vahl (Leguminosae), sold in the market by the name of “tinnevelly senna,” is a variably branched erect shrub, commercially cultivated in southern India. Almost every part (i.e., leaves, pods along with their seeds) is used extensively for its medicinal properties and could be applied for pharmaceutical application (Anonymous, Citation1992). The leaves are considered as astringent, cathartic, depurative, anthelmintic, cholagogue, expectorant, and febrifuge, useful for constipation, leprosy, leukoderma, jaundice, typhoid fever, tumors, and vitiated conditions of “pitta” and “vata” (Warier, Citation1994).
Anthraquinone derivatives are the main active constituents of senna, which are responsible for its laxative properties. Chemically they contained sennosides A, B, C, and D (Christ et al., 1978; Hayashi et al., Citation1980), kaemferol, phytosterols (Khorana & Sanghvi, Citation1964; Dane et al., Citation1972), glycosides of rhein, and chrysophanic acid. Tinnevelin glycoside is also identified in C. angustifolia., which distinguished it from C. auriculata., the most common species used as the drug senna (Lemli, Citation1986). Apart from their leaves, seeds also contained water-soluble polysaccharides (Chaubey & Kapoor, Citation2001).
Arrieta et al. (Citation2002) isolated a peroxidase and concluded that it may participate in the biogenesis of anthraquinone. Cassia angustifolia. extract could regulate disorders of the gastrointestinal tract after abdominal operations (Wang et al., Citation1998). The extracts possess topical anti-inflammatory activity (Cuellar et al., Citation2001) and virucidal affects (Sydiskis et al., Citation1991). Wang et al. (Citation2002) has also identified protein mediating senna-induced gastrointestinal motility enhancement in mucous colon.
The leaves containing sennosides are efficient sources of health teas (Kojima et al., Citation2001) and are available singly or in different herbal formulations in the drug markets (e.g., Herbolax, Periderm granules, Senolax, and Verechni tablets).
Seeds possess a diversified medicinal and economic importance, and no detailed pharmacognostical studies of the seeds of Cassia angustifolia. is on record. In this context, the pharmacognostical evaluation of C. angustifolia. seeds was performed to establish quality control of the seed. Further, the samples were also procured from different parts of the country to study the geographical variations, if any.
Materials and Methods
The seeds of Cassia angustifolia. (about 500 g each) were procured from four different places in India, viz., DehraDun (LWG 221241), Hyderabad (LWG 221242), Jodhpur (LWG 221243), and Mumbai (LWG 221244), and matched with the herbarium specimens and deposited in the institute's herbal drug museum depository. The collected samples were dried at 40–50°C. The transverse sections were cut for microscopic details, and histochemical evaluation of various contents present in seeds was studied according to Johansen (Citation1940). The color changes of the powdered seeds with respect to different chemical reagents on the basis of different chemical constituents was observed in day and ultraviolet light (UV 254 nm and 366 nm) as per methods described by Chase and Pratt (Citation1949) and Kokoski et al. (Citation1958).
The percentage of physicochemical values, viz., moisture content, total ash, acid insoluble ash, water- and alcohol-soluble extractives, percentage mucilage and fiber content were calculated according to methods described in the Indian Pharmacopoeia. (Anonymous, Citation1966). The percentage of protein (Lowry et al., Citation1951), sugar/starch (Montgomery, Citation1957), and tannin (Anonymous, Citation1984) was also determined using a Pharma Biotech 2000 spectrophotometer.
For HPTLC, 0.5 g of powdered seed samples from four different regions (DehraDun, Hyderabad, Jodhpur, and Mumbai) were refluxed separately for 5 min on water bath with 5 ml of 50% methanol and filtered. The filtrate was concentrated and the extract was made in a known volume. The filtrate of each test solution (25 µl) was then applied on precoated silica gel 60-F254 Merck TLC glass plates of 10 × 10 cm with the help of a Camag Linomat IV applicator. Sennoside A (0.1 mg) and sennoside B (0.55 mg) in isopropyl alcohol were also applied adjacent to the seed samples. The plate was developed to a distance of 8.7 cm at room temperature (33°C) in the solvent system toluene:ethyl acetate:methanol (85:15:0.5). It was sprayed with anisaldehyde–sulfuric acid reagent, heated at 120°C for 10 min (Wagner et al., 1984), and a fingerprint profile was obtained with the help of a Desaga Video Documentation Unit (Providoc II). Quantitative estimation of sennosides A and B were done using a Camag densitometric scanner.
Results and Discussion
Brief taxonomic description of the plant
Cassia angustifolia. is a small perennial undershrub, below 1 m in height with ascending branches. It attains 0.7–1.0 m height under cultivation but sometimes grows taller (1.5 m). It sheds the leaves with the onset of cold weather in northern India. The plant bears compound, pinnate leaves, having 5 to 8 pairs of leaflet. The leaflets are oval-lanceolate 2 to 5 × 1.5 cm, pale-green, glabrous with smooth margin, acute apex, and oblique base. It usually comes in flowering at 65–70 days age (after sowing) and produces 30–45 cm long axillary or subterminal racemes bearing many large brilliant yellow-colored showy flowers (). The pods are pale-green in the beginning, which change to greenish-brown and dark-brown on maturity and after drying. They are flat, thin, 3.5 to 8.5 cm × 1.5 cm in dimension, containing 5–8 obovate yellow to off-cream–colored flat seeds.
Description of seed
Macromorphology
The seeds are creamish-brown, wedge-shaped with transverse ridges and furrows on the surface and have prominent hilum, micropyle, and raphe. The hilum is large oval in shape and represented by a scar near the middle of the edge, the micropyle is small, and the raphe is ridged at the side of hilum opposite to the micropyle and is elongated and arced ().
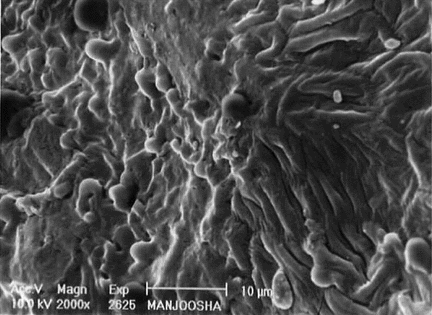
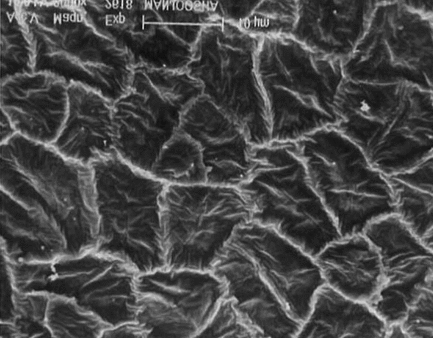
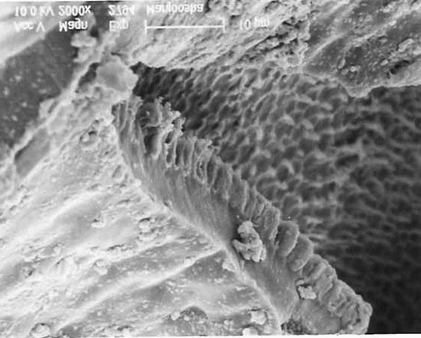
SEM studies
On scanning electron microscopic studies , it was observed that the cells of the seed coat are penta to hexagonal in shape, the margins are frilled, and surface of cells are flushed with small irregular wrinkles. The lens is long blunt with thick raised and undulated boundaries, a slight depression is observed toward the distal end with prominent elongated hypocotyls, which are slightly sinuous. The transverse lines are also observed on the surface of the hypocotyl. The surface of endosperm in magnified view of 2000× showed wax deposition. The surface of cotyledon is honeycomb-like with irregular folds. The distal end of cotyledons is papillate with prominent undulated boundaries.
Micromorphology
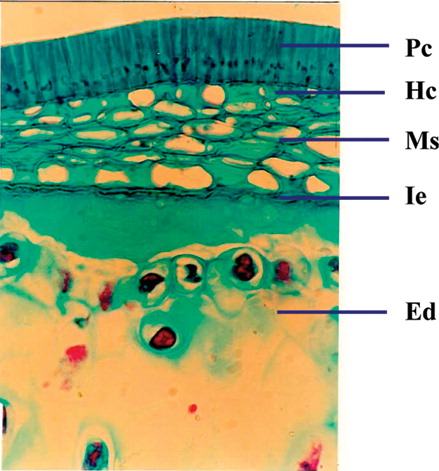
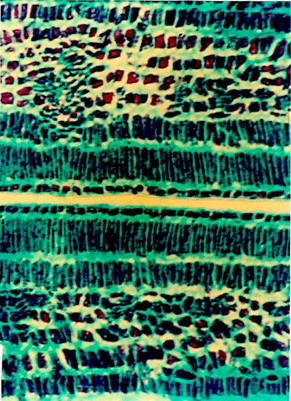
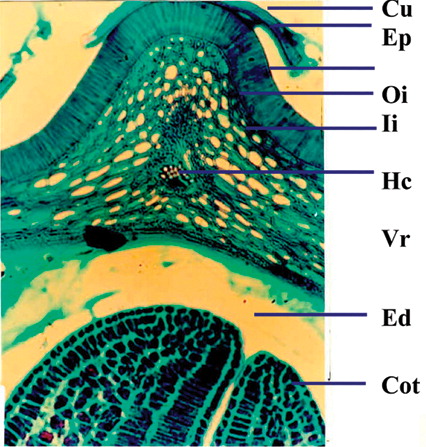
A cross section of seed coat through hilar region showed a single layer of cuticle followed by single layered epidermis (). The palisade cells derived from the outer epidermis of the outer integument are elongated and closely arranged. Following the palisade layer are situated the characteristic bottle-shaped hourglass cells. The remainder of the testa is mainly composed of mesophyll cells. Near the hilum these cells are small and compressed. The linea lucida is discernible in the lower half of the palisade cells. The vascular supply is prominent in the middle of the mesophyll cell. The cotyledons are composed of numerous parenchymatous cells filled with starch grains. The size of the parenchymatous cell ranges from 70 to 100 µm. Each parenchymatous cell is bounded by a distinct cell wall with the middle lamella.
Figure 7 Cellular structure of seed showing micropyle and cotyledons. Cu, cuticle; Ep, epidermis; Oi, outer integument; Ii, inner integument; Hc, hourglass cells; Vr, vascular regions; Ed, endosperm; Cot, cotyledons; Pc, palisade-like cells; Ms, mesophyll; Ie, inner epidermis.
Powder studies
Powder when treated with different chemical reagents showed the color reactions tabulated in .
Table 1 Powder studies in seed of C. angustifolia..
Physico-chemical studies
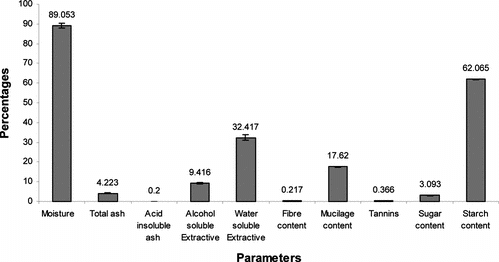
The physicochemical parameters of the seed, viz., percentage moisture content, total ash, and acid insoluble ash were found to be 89.05%, 4.20%, and 0.20%, respectively. Percent alcohol extractive was 9.40, but the percentage of water extractive was found to be significantly higher (i.e., 32.40% in the seed). Percentage starch content in the seeds was very high (i.e., up to 62.00%), whereas percentage sugar was limited to just 3.00%. Seeds also possessed mucilage content of 17.62%, while percent fiber and tannin was 0.366% and 0.217%, respectively ().
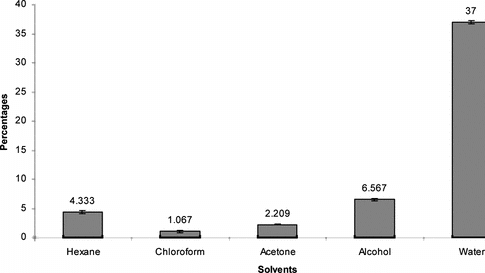
Successive Soxhlet extractive values of the seed, viz., hexane chloroform, acetone, alcohol, and water, were also determined and found to be 4.33%, 1.07%, 2.209%, 6.56%, and 37%, respectively ().
HPTLC studies
HPTLC studies were performed to develop characteristic gross HPTLC fingerprint profile, which may be used as marker for quality evaluation and standardization of the drug (). In this study, eight chemical marker components were determined at a Rf of 0.10, 0.21, 0.27, 0.32, 0.52, 0.58, 0.62, and 0.64 of characteristic color in all the seed samples procured from different geographical zones of the country.
Figure 13 HPTLC fingerprint profile of different samples of Cassia angustifolia. seeds procured from different regions (1–4) along with the active constituents sennoside A and B.
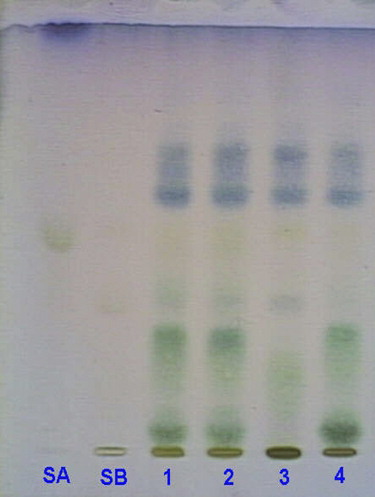
Sennosides are the main active components in C. angustifolia.. Various researchers have estimated their concentration in the leaves and pericarp of the fruit, but no such record is available for seeds. Because in the current study the main emphasis was on medicinal and commercial value of seeds, the percentage of sennosides was also determined in seeds, and it was found that the concentration of sennoside B was high in the majority of the samples. The plate in UV 254 and visible light after spraying with detecting reagent showed the presence of sennoside A and sennoside B at Rf of 0.52 and 0.32, respectively, in all of the seed samples. Sennoside A and B, when qualitatively estimated, were found to be present in all the seed samples though their quantity varied from one region to another, possibly due to geographical variations ().
The effect of different geographical zones on the concentration of sennosides (A and B) was also studied. The concentration of sennoside A varied from 0.21% to 2.13% in seeds of different regions, while sennoside B showed a wide range of variation from 1.34% to 8.52%. Hyderabad seeds possessed a maximum of sennoside A (2.13%), which was least in the Bombay seeds, limited to just 0.219%. On the contrary, sennoside B was found to be maximum in the case of Jodhpur seeds (8.52%), which was about 7-times more than the seeds of Bombay, (i.e., 1.34%). Hyderabad seeds also possessed an appreciable concentration of sennoside B, 7.4%, while in Dehradun seeds the concentration was 3.95% ().
Table 2 Percentage concentration of sennoside A and B in seed samples of different geographic regions.
Conclusion
C. angustifolia. seeds are identified and authenticated through macroscopic and microscopic studies, which is further confirmed by similar HPTLC profiles. Quantification of the sennosides A and B showed that they varied from 1.00% to 2.14% and 1.34% to 3.95%, respectively, from one geographic location to another. Furthermore, the seeds contained sufficient starch and mucilage to be exploited commercially for economic use.
Acknowledgment
The authors are thankful to the director of NBRI for providing facilities and encouragement throughout the work.
References
- Anonymous (1966): Indian Pharmacopoeia, 2nd ed. New Delhi, Government of India Publication, pp. 367–370.
- Anonymous (1984): Official Methods of Analysis (AOAC) Virginia., 14th ed. In: K. Helrich. Association of Official Analytical Chemists Inc., pp. 1010–1039.
- Anonymous (1992): The Wealth of India II-Raw Materials. New Delhi, Publication and Information Directorate, CSIR, pp. 354–363.
- Arrieta-Baez D, Roman R, Vazquez-Duhalt R, Jimenez-Estrada M (2002): Peroxidase-mediated transformation of hydroxy-9,10-anthraquinones. Phytochemistry 60: 567–572, [INFOTRIEVE], [CSA]
- Ceuellar MJ, Giner RM, Recio MC, Manez S, Rios JL (2001). Topical anti-inflamatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia 72: 221–229, [CROSSREF], [CSA]
- Chase CR, Pratt RJ (1949): Fluorescence of powdered vegetable drugs with particular reference to development of a system of identification. J Am Pharm Assoc 38: 324–331, [CSA]
- Chaubey M, Kapoor VP (2001): Structure of galactomannan from the seeds of Cassia angustifolia. Vahl. Carbohydr Res 332: 439–444, [INFOTRIEVE], [CROSSREF], [CSA]
- Dane VB, Deshmukh VK, Saoji AN (1972): A study of development of glycosides during growth in Cassia angustifolia. pods. Indian J Pharm 34: p. 169, [CSA]
- Hayashi S, Yoshika A, Tanaka H, Mitani Y, Yoshizawa K (1980): Analytical studies on the active constituent in crude drug-IV. Determination of sennosides in Senna and its formulations by HPLC. Chem Pharm Bull 28: 406–412, [INFOTRIEVE], [CSA]
- Johansen DA (1940): Plant Micro Techniques 182.. New York, McGraw-Hill, pp. 89–93.
- Khorana ML, Sanghavi MM (1964): Two new glucosides from Cassia angustifolia. pods. J Pharm Sci 53: 110–113, [INFOTRIEVE], [CSA]
- Kojima T, Kishi M, Sekita S, Satake M (2001): Origin of sennosides in health teas including Malva. leaves. Shokuhin Eiseigaku Zasshi 42: 202–205, [INFOTRIEVE], [CSA]
- Kokoski J, Kokoski R, Slama FJ (1958). Fluorescence of powdered vegetable drugs under ultraviolet radiation. J Am Pharmacol Assoc 47: 75–78, [CSA]
- Lemli J (1986): The chemistry of Senna. Fitoterapia LVII(1): 33–38, [CSA]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with Folin phenol reagent. J Biol Chem 193: 266–265, [CSA]
- Montgomery R (1957): Determination of glycogen. Arch Biochem Biophys 67: 378–386, [INFOTRIEVE], [CROSSREF], [CSA]
- Sydiskis RJ, Owen DG, Lohr JL, Rosler KH, Blomster RN (1991): Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Aegnts Chemother 35: 2463–2466, [CSA]
- Wagner H, Bladt S (1996): Plant Drug Analysis, 2nd ed. Berlin, Heidelberg, Spring Verlag, pp. 237–239.
- Wang M, Yan S, Wang J (1998): Clinical and experimental study on Cassia angustifolia. extract as enema after abdominal operations. Zhongguo Zhong Xi Yi Jie He Za Zhi 18: 540–542, [INFOTRIEVE], [CSA]
- Wang X, Zhong YX, Lancaster M, Zhang ZY, Shi YQ, Lu J, Ding J, Wu KC, Jin JP, Pan BR, Fan DM (2002): Screening and identification of protein mediating senna induced gastrointestinal motility enhancement in mouse colon. World J Gastroenterol 8: 162–167, [INFOTRIEVE], [CSA]
- Warier, PS (1994): Indian Medicinal Plants, Vol. 2. New Delhi, Orient Longman Publication, p. 31.