Abstract
A series of coumarins and related compounds were synthesized and screened as potential anti-tumor-promoting agents by examining the ability of the compound to inhibit Epstein-Barr virus early antigen (EBV-EA) activation induced by 12-O.-tetradecanoylphorbol-13-acetate (TPA) in Raji cells. The most promising compound in this in vitro. assay, 7,8-di(3-methyl-2-butenyloxy)coumarin (17), showed confirmed chemopreventive activity in an in vivo. two-stage assay of mouse skin tumors (DMBA/TPA). This investigation also confirmed an important role for the prenyl moiety, and, possibly, for the dimethylpyran substructure, on the anti-tumor-promoting activity.
Introduction
Many kinds of anti-tumor agents have been discovered and developed; however, cancer currently remains a tragic disease, and side effects of the anti-tumor agents are a serious and critical problem in the treatment of cancer. The mechanism of chemical carcinogenesis has been explained by either a two-stage theory or a multistage theory, which consists of initiation, promotion, and progression stages (Sielken et al., Citation1994). Among these stages, the promotion stage is long-term and consists of reversible reactions; thus, the development of inhibitors of the promotion stage has been regarded as one of the most effective methods for the inhibition of carcinogenesis. Such inhibitors are called anti-tumor-promoting (cancer chemopreventive) agents (Konoshima Takasaki, Citation2000; Tsao et al., Citation2004). Since 1987, there has been an active search for anti-tumor-promoting agents from natural resources (Lai Roy, Citation2004; Sengupta et al., Citation2004). Several compounds such as glycyrrhetic acid (O'Brian et al., Citation1990; Konoshima et al., Citation1996), curcuminoids (Narayan, Citation2004), and β-carotene (Nishinio et al., Citation2002; Kristal, Citation2004) have been reported as potent anti-tumor-promoting agents.
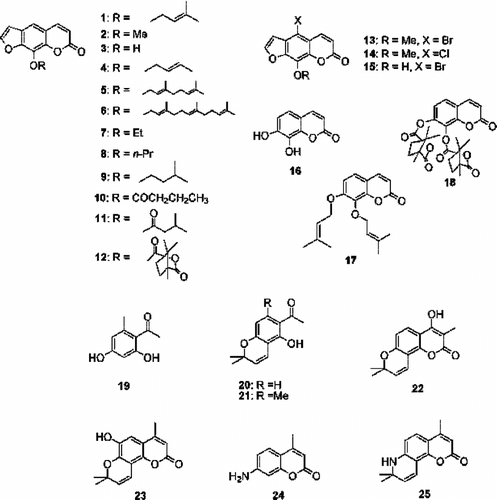
Several reports have concerned anti-tumor-promoter effects of coumarins (Murakami et al., Citation2000; Itoigawa et al., Citation2001; Kleiner et al., Citation2002; Akihisa et al., Citation2003; Ito et al., Citation2005; Ju-Ichi, Citation2005). Consequently, we were prompted to perform a structure-activity relationship (SAR) study of synthetic coumarin and were particularly interested in the anti-tumor-promoting activity of imperatorin (1) (Kleiner et al., Citation2002). More than 20 coumarins and related intermediates/compounds () were synthesized and their activity investigated using an in vitro. Epstein-Barr virus (EBV) early antigen activation in Raji cells, induced by the tumor promoter 12-O.-tetradecanoylphorbol-13-acetate (TPA). The coumarin with the most promising activity (17) was selected and studied in vivo. for chemoprevention of 7,12-dimethylbenz[a.]anthracene (DMBA)-induced TPA-promoted mouse skin carcinogenesis. The results showed that this compound is a potent inhibitor of skin tumor promotion in mice treated with DMBA and TPA.
Materials and Methods
Chemistry
8-Methoxypsoralens, 2 and 13, respectively, were demethylated using BBr3 in dichloromethane at 0 °C to yield 3 and 5. Psoralen 3 was allylated by reacting with the appropriate allyl bromide and potassium carbonate in dry acetone to give 1, 4, and 6. Compounds 7–12 were prepared by alkylating or acylating psolaren 3 with the appropriate alkyl halide or acyl halide, potassium carbonate, and crown ether in dry acetone. Compound 2 was reacted with bromine in glacial acetic acid and with N.-chlorosuccinimide in refluxing carbon tetrachloride solution to give 13 and 14, respectively. Compound 16 was reacted with alkyl/acyl halide, potassium carbonate, without or with crown ether, to provide 17 and 18, respectively. Compound 19 was prepared by acylating orsinol with acetyl chloride and aluminum chloride in nitrobenzene. Compounds 20, 21, and 23 were synthesized by reacting 4,6-dihydroxy acetophenone, compound 19, 4-methyl-6,7-dihydroxycoumarin, respectively, with 3-hydroxy-3-methyl-1,1-dimethoxybutanol in dry pyridine at 150 °C. 1-(5-Hydroxy-2,2-dimethyl-2H.-chromen-6-yl)-propan-1-one was reacted with refluxing diethyl carbonate and sodium hydride to give 22. To obtain 25, compound 24 was reacted first with 3-chloro-3-methylbut-1-yne, copper powder, and copper chloride (I) in refluxing acetone (50% aq.), then the obtained intermediate was cyclized in the presence of copper chloride in refluxing THF. Compounds 3, 5, 16, and 24 were commercially available.
In vitro. EBV-EA activation assay
This assay was performed using Raji cells (virus non-producer type), an EBV genome-carrying human lymphoblastoid cell, which were cultivated in 10% fetal bovine serum (FBS) RPMI 1640 medium. The indicator cells (Raji, 1 × 106/ml) were incubated at 37°C for 48 h in 1 ml of medium containing n.-butyric acid (4 mM, inducer), TPA (32 pM), and various amounts of test compounds dissolved in 5 µl of DMSO. Smears were made from the cell suspension. The EBV-EA inducing cells were stained with high-titer EBV-EA positive serum from NPC patients and detected by an indirect immunofluorescence technique. In each assay, 500 cells were counted, and the number of stained cells (positive cells) was recorded. Triplicate assays were performed for each data point. The EBV-EA inhibitory activity of the test compounds was expressed by comparison with that of the positive control experiment (100%), which was carried out with n.-butyric acid (4 mM) plus TPA (32 pM). In the experiments, the EBV-EA induction was ordinarily around 35%, and this value was taken as the positive control (100%). n.-Butyric acid (4 mM) alone induced 0.1% EA-positive cells.
Cytotoxicity determination
For the determination of the cytotoxicity or cell viability of surviving cells, the Trypan blue staining method was used. After EBV-EA activating assays, 0.1 ml of treated cells (suspended in PBS) was stained with 0.1 ml of 0.25% Trypan blue solution. Dead cells were stained blue. Viable unstained cells were counted.
In vivo. carcinogenesis assay
For two-stage mouse skin carcinogenesis test, 6-week-old female SENCAR mice were used. Each group of 15 mice was housed 5 per cage. The basal diet and tap water were available ad libitum. throughout the experiment. The back of each mouse was shaved with surgical clippers. Each mouse was treated topically with 390 nmol DMBA in 0.1 ml acetone. After 1 week, each mouse topically received 1.7 nmol TPA in 0.1 ml acetone twice a week for 20 weeks. One hour before TPA treatment, the mice were treated with the test compound (85 nM) in acetone. The mice were examined weekly for the incidence of papillomas during the 20 weeks of the follow-up period.
Results and Discussion
In the in vitro. assay, most tested compounds showed potent inhibition of EBV activation that was comparable or better than glycyrrhetic acid, a known anti-tumor-promoter (). Compounds 1, 4, 17, 20, and 21 showed inhibitory activity even at 1 × 10 mol ratio/TPA concentration. In particular, compound 17, which has two prenyl side chains, exhibited the highest potency (75%, 43%, 11% inhibition of activation at 5 × 102, 1 × 102, 1 × 10 mol ratio/TPA, respectively).
Table 1 Inhibitory effects of coumarins and derivatives on TPA-mediated EBV-EA induction.
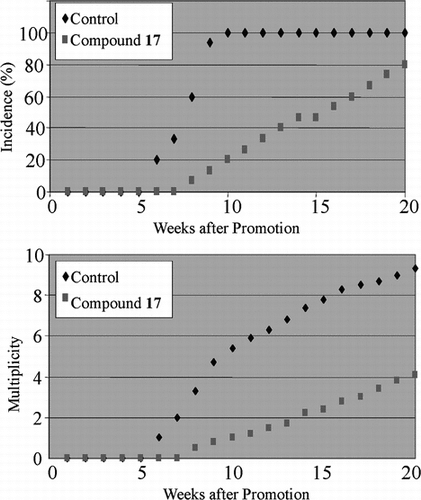
For the most active compound (17), an in vivo. study was conducted. The results presented in show the incidence of papilloma occurrence (the percentage of mice having papillomas) with and without oral administration of 17 during a period of 20 weeks. The control animals, which received treatment with DMBA and TPA, but without 17, showed a 100% incidence of papillomas in less than 10 weeks. The test animals took 20 weeks to show even 80% papilloma formation. A tumor inhibitory effect is also seen by a reduction in the multiplicity of papillomas (the number of papillomas formed per mouse) during the 20-week period, another indication of the inhibitory potential of 17 toward DMBA- and TPA-induced two-stage skin carcinogenesis.
The high activity of psoralen derivatives 1 and 4, which are modified with prenyl or crotyl, respectively, corresponds with the earlier findings that O.-allyl moieties in phenylpropanoids play an important role in the inhibition of EBV-EA induction (Ito et al., Citation2003; Itoigawa et al., Citation2004). On the other hand, 5 and 6, compounds containing the extended prenyl units, geranyl, and farnesyl, respectively, were less active. Compounds 9–11,which contain isopentyl, butanoyl, and isovaleryl groups, respectively, showed similar activity profiles to 1. Therefore, the length of the alkyl group may be more important than the presence of unsaturation. Compounds 3, 7, and 8 with hydroxy, ethoxy, and propyloxy substituents, respectively, at the 8-position were less active, although 8-methoxypsoralen (2, xanthothoxin) showed good activity at the highest concentration. Halogenated methoxypsoralens 13 and 14 were almost three-fold less active than 2.
With respect to the inhibitory activity of seselins and synthetic intermediates, 6-acetyl-2H.-chromenes 20 and 21 without the pyranone ring showed high potency (96%, 60%, and 30% inhibition of activation at 1 × 103, 5 × 102, and 1 × 102 mol ratio/TPA, respectively). The acetophenone 19 and the coumarins 16 and 24 were less active, suggesting the importance of the dimethylpyran moiety, which is a biosynthetically rearranged prenyl unit. Coumarins 22, 23, and 25 with a dimethylpyran ring showed slightly improved activity but, were not as potent as acetophenones.
Conclusion
In summary, not only compounds 1 and 4 with a linear prenyl or prenyl-like (crotyl) unit but also 20 and 21 with a cyclized prenyl unit showed potent chemopreventive properties. Compound 17 with two separate prenyl units exhibited the most potent chemopreventive activity, whereas 5 and 6 with extended prenyl units were much weaker. Many compounds exhibited in vitro. chemopreventive effects better or comparable with the positive control β.-carotene. In addition, chemopreventive activity of the most potent coumarin 17 was confirmed in vivo.. This first systematic SAR study on the chemopreventive activity of coumarins offers new insights for possible development of potent new chemopreventive agents.
Acknowledgment
This investigation was supported in part by a NIH grant CA-17625 from the National Cancer Institute awarded to K.H. Lee.
References
- Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H (2003): Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei. and their chemopreventive effects. Cancer Lett 201: 133–137, [INFOTRIEVE], [CSA]
- Ito C, Itoigawa M, Miyamoto Y, Onoda S, Rao KS, Mukainaka T, Tokuda H, Nishino H, Furukawa H (2003): Polyprenylated benzophenones from Garcinia assigu. and their potential cancer chemopreventive activities. J Nat Prod 66: 206–209, [INFOTRIEVE], [CROSSREF], [CSA]
- Ito C, Itoigawa M, Ju-ichi M, Sakamoto N, Tokuda H, Nishino H, Furukawa H (2005): Antitumor-promoting activity of coumarins from citrus plants. Planta Med 71: 84–87, [INFOTRIEVE], [CROSSREF], [CSA]
- Itoigawa M, Ito C, Tan HT, Kuchide M, Tokuda H, Nishino H, Furukawa H (2001): Cancer chemopreventive agents, 4-phenylcoumarins from Calophyllum inophyllum.. Cancer Lett 169: 15–19, [INFOTRIEVE], [CROSSREF], [CSA]
- Itoigawa M, Ito C, Tokuda H, Enjo F, Nishino H, Furukawa H (2004): Cancer chemopreventive activity of phenylpropanoids and phytoquinoids from Illicium. plants. Cancer Lett 214: 165–169, [INFOTRIEVE], [CROSSREF], [CSA]
- Ju-Ichi M (2005): Chemical study of citrus plants in the search for cancer chemopreventive agents. Yakugaku Zasshi 125: 231–254, [INFOTRIEVE], [CROSSREF], [CSA]
- Kleiner HE, Vulimiri SV, Starost MF, Reed MJ, DiGiovanni J (2002): Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a.]anthracene in SENCAR mice. Carcinogenesis 23: 1667–1675, [INFOTRIEVE], [CROSSREF], [CSA]
- Konoshima T, Takasaki M (2000): Anti-tumor-promoting activities (cancer chemopreventive activities) of natural products. Studies Nat Prod Chem 24: 215–267, [CSA]
- Konoshima T, Takasaki M, Tokuda H, Masuda K, Arai Y, Shiojima K, Ageta H (1996): Anti-tumor-promoting activities of triterpenoids from ferns. I. Biol Pharm Bull 19: 962–965, [INFOTRIEVE], [CSA]
- Kristal AR (2004): Vitamin A, retinoids and carotenoids as chemopreventive agents for prostate cancer. J Urol 171: S54–S58, [INFOTRIEVE], [CROSSREF], [CSA]
- Lai PK, Roy J (2004): Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem 11: 1451–1460, [INFOTRIEVE], [CSA]
- Murakami A, Nakamura Y, Tanaka T, Kawabata K, Takahashi D, Koshimizu K, Ohigashi H (2000): Suppression by citrus auraptene of phorbol ester-and endotoxin-induced inflammatory responses: Role of attenuation of leukocyte activation. Carcinogenesis 21: 1843–1850, [INFOTRIEVE], [CROSSREF], [CSA]
- Narayan SV (2004): Curcumin, a multi-functional chemopreventive agent, blocks growth of colon cancer cells by targeting beta-catenin-mediated transactivation and cell-cell adhesion pathways. J Mol Histol 35: 301–307, [INFOTRIEVE], [CROSSREF], [CSA]
- Nishino H, Murakoshi M, Ii T, Takemura M, Kuchide M, Kanazawa M, Mou XY, Wada S, Masuda M, Ohsaka Y, Yogosawa S, Satomi Y, Jinno K (2002): Carotenoids in cancer chemoprevention. Cancer Metastasis Rev 21: 257–264, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Brian CA, Ward NE, Vogel VG. (1990): Inhibition of protein kinase C by the 12-O.-tetradecanoylphorbol-13-acetate antagonist glycyrrhetic acid. Cancer Lett 49: 9–12, [INFOTRIEVE], [CROSSREF], [CSA]
- Sengupta A, Ghosh S, Bhattacharjee S, Das S (2004): Indian food ingredients and cancer prevention—an experimental evaluation of anticarcinogenic effects of garlic in rat colon. Asian Pac J Cancer Prev 5: 126–132, [INFOTRIEVE], [CSA]
- Sielken RL Jr, Bretzlaff RS, Stevenson DE (1994): Incorporating additional biological phenomena into two-stage cancer models. Prog Clin Biol Res 387: 237–260, [INFOTRIEVE], [CSA]
- Tsao AS, Kim ES, Hong WK (2004): Chemoprevention of cancer. CA Cancer J Clin 54: 150–180, [INFOTRIEVE], [CSA]